Systematic review and meta-analysis of tocilizumab in persons with coronavirus disease-2019 (COVID-19)
- PMID: 34002026
- PMCID: PMC8127467
- DOI: 10.1038/s41375-021-01264-8
Systematic review and meta-analysis of tocilizumab in persons with coronavirus disease-2019 (COVID-19)
Abstract
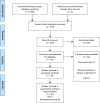
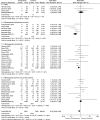
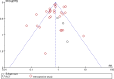
We performed a meta-analysis to determine safety and efficacy of tocilizumab in persons with coronavirus disease-2019 (COVID-19). We searched PubMed, Web of Science and Medline using Boolean operators for studies with the terms coronavirus OR COVID-19 OR 2019-nCoV OR SARS-CoV-2 AND tocilizumab. Review Manager 5.4 was used to analyze data and the modified Newcastle-Ottawa and Jadad scales for quality assessment. We identified 32 studies in 11,487 subjects including three randomized trials and 29 cohort studies with a comparator cohort, including historical controls (N = 5), a matched cohort (N = 12), or concurrent controls (N = 12). Overall, tocilizumab decreased risk of death (Relative Risk [RR] = 0.74; 95% confidence interval [CI], 0.59, 0.93; P = 0.008; I2 = 80%) but not of surrogate endpoints including ICU admission (RR = 1.40 [0.64,3.06]; P = 0.4; I2 = 88%), invasive mechanical ventilation (RR = 0.83 [0.57,1.22]; P = 0.34; I2 = 65%) or secondary infections (RR = 1.30 [0.97,1.74]; P = 0.08; I2 = 65%) and increased interval of hospitalization of subjects discharged alive(mean difference [MD] = 2 days [<1, 4 days]; P = 0.006; I2 = 0). RRs of death in studies with historical controls (RR = 0.28 [0.16,0.49; P < 0.001]; I2 = 62%) or a matched cohort (RR = 0.68 [0.53, 0.87]; P = 0.002; I2 = 42%) were decreased. In contrast, RRs of death in studies with a concurrent control (RR = 1.10 [0.77, 1.56]; P = 0.60; I2 = 85%) or randomized (RR = 1.18 [0.57,2.44]; P = 0.66; I2 = 0) were not decreased. A reduced risk of death was not confirmed in our analyses which questions safety and efficacy of tocilizumab in persons with COVID-19.
Conflict of interest statement
RPG is a consultant to: BeiGene Ltd., Fusion Pharma LLC, LaJolla NanoMedical Inc., Mingsight Parmaceuticals Inc. and CStone Pharmaceuticals. Advisor: Antegene Biotech LLC, Medical Director: FFF Enterprises Inc. Partner: AZACA Inc. Board of Directors: RakFond Foundation for Cancer Research Support. Scientific Advisory Board, StemRad Ltd. All other authors declare no competing interests.
Figures



References
-
- Canziani LM, Trovati S, Brunetta E, Testa A, De Santis M, Bombardieri E, et al. Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: a retrospective case-control survival analysis of 128 patients. J of autoimmun. 2020;114:102511. - PMC - PubMed
-
- Kaur S, Bansal Y, Kumar R, Bansal G. A panoramic review of IL-6: structure, pathophysiological roles and inhibitors. Bioorg & med chem. 2020;28:115327. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

