Kinase drug discovery 20 years after imatinib: progress and future directions
- PMID: 34002056
- PMCID: PMC8127496
- DOI: 10.1038/s41573-021-00195-4
Kinase drug discovery 20 years after imatinib: progress and future directions
Abstract
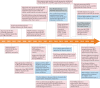
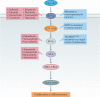
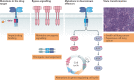
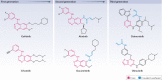
Protein kinases regulate nearly all aspects of cell life, and alterations in their expression, or mutations in their genes, cause cancer and other diseases. Here, we review the remarkable progress made over the past 20 years in improving the potency and specificity of small-molecule inhibitors of protein and lipid kinases, resulting in the approval of more than 70 new drugs since imatinib was approved in 2001. These compounds have had a significant impact on the way in which we now treat cancers and non-cancerous conditions. We discuss how the challenge of drug resistance to kinase inhibitors is being met and the future of kinase drug discovery.
Conflict of interest statement
P.C. has shares in Alliance Pharma, AstraZeneca and GlaxoSmithKline and is a member of the Scientific Advisory Boards of Mission Therapeutics, Ubiquigent and Biocatalyst International. D.C. is an employee and shareholder of AstraZeneca. P.A.J. has received consulting fees from AstraZeneca, Boehringer-Ingelheim, Pfizer, Roche/Genentech, Takeda Oncology, ACEA Biosciences, Eli Lilly and Company, Araxes Pharma, Ignyta, Mirati Therapeutics, Novartis, LOXO Oncology, Daiichi Sankyo, Sanofi Oncology, Voronoi, SFJ Pharmaceuticals, Biocartis, Novartis Oncology, Nuvalent, Esai, Bayer, Transcenta and Silicon Therapeutics; receives post-marketing royalties from DFCI-owned intellectual property on
Figures
References
-
- Haeder M, et al. Epidermal growth factor receptor expression in human lung cancer cell lines. Cancer Res. 1988;48:1132–1136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

