UCP1 governs liver extracellular succinate and inflammatory pathogenesis
- PMID: 34002097
- PMCID: PMC8207988
- DOI: 10.1038/s42255-021-00389-5
UCP1 governs liver extracellular succinate and inflammatory pathogenesis
Abstract
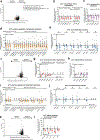
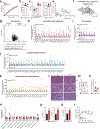
Non-alcoholic fatty liver disease (NAFLD), the most prevalent liver pathology worldwide, is intimately linked with obesity and type 2 diabetes. Liver inflammation is a hallmark of NAFLD and is thought to contribute to tissue fibrosis and disease pathogenesis. Uncoupling protein 1 (UCP1) is exclusively expressed in brown and beige adipocytes, and has been extensively studied for its capacity to elevate thermogenesis and reverse obesity. Here we identify an endocrine pathway regulated by UCP1 that antagonizes liver inflammation and pathology, independent of effects on obesity. We show that, without UCP1, brown and beige fat exhibit a diminished capacity to clear succinate from the circulation. Moreover, UCP1KO mice exhibit elevated extracellular succinate in liver tissue that drives inflammation through ligation of its cognate receptor succinate receptor 1 (SUCNR1) in liver-resident stellate cell and macrophage populations. Conversely, increasing brown and beige adipocyte content in mice antagonizes SUCNR1-dependent inflammatory signalling in the liver. We show that this UCP1-succinate-SUCNR1 axis is necessary to regulate liver immune cell infiltration and pathology, and systemic glucose intolerance in an obesogenic environment. As such, the therapeutic use of brown and beige adipocytes and UCP1 extends beyond thermogenesis and may be leveraged to antagonize NAFLD and SUCNR1-dependent liver inflammation.
Conflict of interest statement
Figures



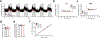



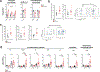

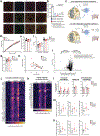


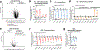
Comment in
-
New BAT-liver endocrine pathway via succinate.Nat Rev Endocrinol. 2021 Aug;17(8):449. doi: 10.1038/s41574-021-00518-w. Nat Rev Endocrinol. 2021. PMID: 34099906 No abstract available.
-
Protective succinate-SUCNR1 metabolic stress signaling gone bad.Cell Metab. 2021 Jul 6;33(7):1276-1278. doi: 10.1016/j.cmet.2021.06.009. Cell Metab. 2021. PMID: 34233171
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

