Long-term follow-up after ultrathin vs. conventional 2nd-generation drug-eluting stents: a systematic review and meta-analysis of randomized controlled trials
- PMID: 34002202
- PMCID: PMC8282325
- DOI: 10.1093/eurheartj/ehab280
Long-term follow-up after ultrathin vs. conventional 2nd-generation drug-eluting stents: a systematic review and meta-analysis of randomized controlled trials
Abstract
Aims: Contemporary 2nd-generation thin-strut drug-eluting stents (DES) are considered standard of care for revascularization of patients undergoing percutaneous coronary intervention. A previous meta-analysis of 10 randomized controlled trials (RCTs) with 11 658 patients demonstrated a 16% reduction in the 1-year risk of target lesion failure (TLF) with ultrathin-strut DES compared with conventional 2nd-generation thin-strut DES. Whether this benefit is sustained longer term is not known, and newer trial data may inform these relative outcomes. We therefore sought to perform an updated systematic review and meta-analysis of RCTs comparing clinical outcomes with ultrathin-strut DES (≤70 µm strut thickness) with conventional 2nd-generation thin-strut DES.
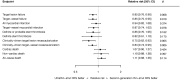
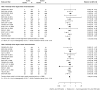
Methods and results: We performed a random-effects meta-analysis of all RCTs comparing ultrathin-strut DES to conventional 2nd-generation thin-strut DES. The pre-specified primary endpoint was long-term TLF, a composite of cardiac death, myocardial infarction (MI), or clinically driven target lesion revascularization (CD-TLR). Secondary endpoints included the components of TLF, stent thrombosis (ST), and all-cause death. There were 16 eligible trials in which 20 701 patients were randomized. The weighted mean follow-up duration was 2.5 years. Ultrathin-strut DES were associated with a 15% reduction in long-term TLF compared with conventional 2nd-generation thin-strut DES [relative risk (RR) 0.85, 95% confidence interval (CI) 0.76-0.96, P = 0.008] driven by a 25% reduction in CD-TLR (RR 0.75, 95% CI 0.62-0.92, P = 0.005). There were no significant differences between stent types in the risks of MI, ST, cardiac death, or all-cause mortality.
Conclusions: At a mean follow-up of 2.5 years, ultrathin-strut DES reduced the risk of TLF, driven by less CD-TLR compared with conventional 2nd-generation thin-strut DES, with similar risks of MI, ST, cardiac death, and all-cause mortality.
Keywords: Coronary artery disease; Drug-eluting stents; Meta-analysis; Percutaneous coronary intervention; Ultrathin-strut.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures





Comment in
-
Meta-analyses of moving targets.Eur Heart J. 2021 Jul 15;42(27):2655-2656. doi: 10.1093/eurheartj/ehab359. Eur Heart J. 2021. PMID: 34115845 Free PMC article. No abstract available.
-
Reporting data from meta-analysis: snapshot of a moving target.Eur Heart J. 2022 Feb 12;43(7):699-700. doi: 10.1093/eurheartj/ehab681. Eur Heart J. 2022. PMID: 34725703 Free PMC article. No abstract available.
References
-
- Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, Doostzadeh J, Cao S, Simonton CA, Sudhir K, Lansky AJ, Cutlip DE, Kereiakes DJ; SPIRIT IV Investigators. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med 2010;362:1663–1674. - PubMed
-
- Schömig A, Dibra A, Windecker S, Mehilli J, Suárez de Lezo J, Kaiser C, Park S-J, Goy J-J, Lee J-H, Di Lorenzo E, Wu J, Jüni P, Pfisterer ME, Meier B, Kastrati AA. Meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J Am Coll Cardiol 2007;50:1373–1380. - PubMed
-
- Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich K-L, Giddings VL, Coleman L, Wong GK, Edelman ER. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation 2011;123:1400–1409. - PMC - PubMed
-
- Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schühlen H, Neumann F-J, Fleckenstein M, Pfafferott C, Seyfarth M, Schömig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 2001;103:2816–2821. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

