A randomized, controlled trial of once-weekly teriparatide injection versus alendronate in patients at high risk of osteoporotic fracture: primary results of the Japanese Osteoporosis Intervention Trial-05
- PMID: 34002252
- PMCID: PMC8563544
- DOI: 10.1007/s00198-021-05996-2
A randomized, controlled trial of once-weekly teriparatide injection versus alendronate in patients at high risk of osteoporotic fracture: primary results of the Japanese Osteoporosis Intervention Trial-05
Erratum in
-
Correction to: A randomized, controlled trial of once-weekly teriparatide injection versus alendronate in patients at high risk of osteoporotic fracture: primary results of the Japanese osteoporosis intervention Trial-05.Osteoporos Int. 2021 Oct;32(10):2143. doi: 10.1007/s00198-021-06066-3. Osteoporos Int. 2021. PMID: 34448885 Free PMC article. No abstract available.
Abstract
In this randomized, controlled trial, treatment with once-weekly subcutaneous injection of teriparatide for 72 weeks was found to be associated with a significant reduction in the incidence of morphometric vertebral fractures compared with alendronate in women with primary osteoporosis who were at high risk of fracture.
Introduction: To determine whether the anti-fracture efficacy of teriparatide is superior to that of alendronate, a prospective, randomized, open-label, blinded-endpoint trial was performed.
Methods: Japanese women aged at least 75 years were eligible for the study if they had primary osteoporosis and were at high risk of fracture. Patients were randomly assigned in a 1:1 ratio to receive sequential therapy (once-weekly subcutaneous injection of teriparatide 56.5 μg for 72 weeks followed by alendronate for 48 weeks) or monotherapy with alendronate for 120 weeks. The primary endpoint was the incidence of morphometric vertebral fractures at 72 weeks (at the end of teriparatide treatment).
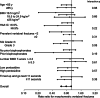
Results: Between October 2014 and December 2017, 1011 patients (505 in the teriparatide group and 506 in the alendronate group) were enrolled. Of these, 778 patients (351 and 427, respectively) were included in the primary analysis. The incidence of morphometric vertebral fractures was significantly lower in the teriparatide group (56 per 419.9 person-years, annual incidence rate 0.1334) than in the alendronate group (96 per 553.6 person-years, annual incidence rate 0.1734), with a rate ratio of 0.78 (95% confidence interval 0.61 to 0.99, P = 0.04). In both groups, adverse events were most frequently reported in the following system organ classes: infections and infestations, gastrointestinal disorders, and musculoskeletal and connective tissue disorders.
Conclusion: Once-weekly subcutaneous injection of teriparatide significantly reduced the incidence of morphometric vertebral fractures compared with alendronate in women with primary osteoporosis who were at high risk of fracture.
Trial registration: jRCTs031180235 and UMIN000015573, March 12, 2019.
Keywords: Bone-forming agent; High risk of fracture; Non-vertebral fracture; Vertebral fracture; Weekly teriparatide.
© 2021. The Author(s).
Conflict of interest statement
H Hagino has received lecture fees or grants outside the submitted work from Amgen Inc., Asahi Kasei Pharma Corp., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan Co., Ltd., Mitsubishi Tanabe Pharma Corp., Mochida Pharma Co., Ltd, Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., and UCB Japan Co., Ltd.
T Sugimoto has received research grants from Asahi Kasei Pharma Corp., Astellas Pharma Inc., Taisho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Pfizer Japan Inc., and Teijin Pharma Co., Ltd., as well as consulting fees from Kissei Pharmaceutical Co., Ltd., Shimadzu Corp., and Takeda Pharmaceutical Co., Ltd.
S Tanaka has received lecture fees from Astra-Zeneca, Taiho Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd.; consultation fees from DeNA Life Science Inc. and CanBus; and outsourcing fees from Satt Co., Ltd. and Asahi Kasei Pharma Corp.
T Sone has received research grants from Astellas Pharma Inc., Eisai Co., Ltd., Daiichi-Sankyo Co., Ltd., Chugai Pharmaceutical Co., Ltd., and Eli Lilly Japan Co., Ltd., as well as consulting and/or lecture fees from Asahi Kasei Pharma Corp., MSD Co., Ltd., and Daiichi-Sankyo Co., Ltd.
T Nakamura has received reports personal fees and other from Asahi Pharma Corp., Teijin Pharma Ltd., Daiichi-Sankyo Pharma Co., Ltd., UCB Japan Co., Ltd., Amgen Inc., Astellas Pharma Inc., and Chugai Pharma Co., Ltd. as well as personal fees and other from Merck & Co., Inc.
S Soen has received consulting fees, speaking fees, and/or honoraria from Amgen Inc., Asahi Kasei Pharma Corp., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan Co., Ltd., Mochida Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., Teijin Pharma Ltd., and UCB Japan Co., Ltd.
K Sasaki and S Mori declare no competing interests.
Figures


References
-
- Eli Lilly and Company (2012) FORTEO highlights of prescribing information. https://pi.lilly.com/us/forteo-pi.pdf. Accessed 26 January 2021
-
- Eastell R, Nickelsen T, Marin F, Barker C, Hadji P, Farrerons J, Audran M, Boonen S, Brixen K, Gomes JM, Obermayer-Pietsch B, Avramidis A, Sigurdsson G, Glüer CC. Sequential treatment of severe postmenopausal osteoporosis after teriparatide: final results of the randomized, controlled European Study of Forsteo (EUROFORS) J Bone Miner Res. 2009;24:726–736. doi: 10.1359/jbmr.081215. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

