Sedatives for opioid withdrawal in newborn infants
- PMID: 34002380
- PMCID: PMC8129634
- DOI: 10.1002/14651858.CD002053.pub4
Sedatives for opioid withdrawal in newborn infants
Abstract
Background: Neonatal abstinence syndrome (NAS) due to opioid withdrawal may result in disruption of the mother-infant relationship, sleep-wake abnormalities, feeding difficulties, weight loss, seizures and neurodevelopmental problems.
Objectives: To assess the effectiveness and safety of using a sedative versus control (placebo, usual treatment or non-pharmacological treatment) for NAS due to withdrawal from opioids and determine which type of sedative is most effective and safe for NAS due to withdrawal from opioids.
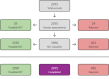
Search methods: We ran an updated search on 17 September 2020 in CENTRAL via CRS Web and MEDLINE via Ovid. We searched clinical trials databases, conference proceedings and the reference lists of retrieved articles for randomised controlled trials and quasi-randomised trials.
Selection criteria: We included trials enrolling infants with NAS born to mothers with an opioid dependence with more than 80% follow-up and using randomised, quasi-randomised and cluster-randomised allocation to sedative or control.
Data collection and analysis: Three review authors assessed trial eligibility and risk of bias, and independently extracted data. We used the GRADE approach to assess the certainty of the evidence.
Main results: We included 10 trials (581 infants) with NAS secondary to maternal opioid use in pregnancy. There were multiple comparisons of different sedatives and regimens. There were limited data available for use in sensitivity analysis of studies at low risk of bias. Phenobarbital versus supportive care: one study reported there may be little or no difference in treatment failure with phenobarbital and supportive care versus supportive care alone (risk ratio (RR) 2.73, 95% confidence interval (CI) 0.94 to 7.94; 62 participants; very low-certainty evidence). No infant had a clinical seizure. The study did not report mortality, neurodevelopmental disability and adverse events. There may be an increase in days' hospitalisation and treatment from use of phenobarbital (hospitalisation: mean difference (MD) 20.80, 95% CI 13.64 to 27.96; treatment: MD 17.90, 95% CI 11.98 to 23.82; both 62 participants; very low-certainty evidence). Phenobarbital versus diazepam: there may be a reduction in treatment failure with phenobarbital versus diazepam (RR 0.39, 95% CI 0.24 to 0.62; 139 participants; 2 studies; low-certainty evidence). The studies did not report mortality, neurodevelopmental disability and adverse events. One study reported there may be little or no difference in days' hospitalisation and treatment (hospitalisation: MD 3.89, 95% CI -1.20 to 8.98; 32 participants; treatment: MD 4.30, 95% CI -0.73 to 9.33; 31 participants; both low-certainty evidence). Phenobarbital versus chlorpromazine: there may be a reduction in treatment failure with phenobarbital versus chlorpromazine (RR 0.55, 95% CI 0.33 to 0.92; 138 participants; 2 studies; very low-certainty evidence), and no infant had a seizure. The studies did not report mortality and neurodevelopmental disability. One study reported there may be little or no difference in days' hospitalisation (MD 7.00, 95% CI -3.51 to 17.51; 87 participants; low-certainty evidence) and 0/100 infants had an adverse event. Phenobarbital and opioid versus opioid alone: one study reported no infants with treatment failure and no clinical seizures in either group (low-certainty evidence). The study did not report mortality, neurodevelopmental disability and adverse events. One study reported there may be a reduction in days' hospitalisation for infants treated with phenobarbital and opioid (MD -43.50, 95% CI -59.18 to -27.82; 20 participants; low-certainty evidence). Clonidine and opioid versus opioid alone: one study reported there may be little or no difference in treatment failure with clonidine and dilute tincture of opium (DTO) versus DTO alone (RR 0.09, 95% CI 0.01 to 1.59; 80 participants; very low-certainty evidence). All five infants with treatment failure were in the DTO group. There may be little or no difference in seizures (RR 0.14, 95% CI 0.01 to 2.68; 80 participants; very low-certainty evidence). All three infants with seizures were in the DTO group. There may be little or no difference in mortality after discharge (RR 7.00, 95% CI 0.37 to 131.28; 80 participants; very low-certainty evidence). All three deaths were in the clonidine and DTO group. The study did not report neurodevelopmental disability. There may be little or no difference in days' treatment (MD -4.00, 95% CI -8.33 to 0.33; 80 participants; very low-certainty evidence). One adverse event occurred in the clonidine and DTO group. There may be little or no difference in rebound NAS after stopping treatment, although all seven cases were in the clonidine and DTO group. Clonidine and opioid versus phenobarbital and opioid: there may be little or no difference in treatment failure (RR 2.27, 95% CI 0.98 to 5.25; 2 studies, 93 participants; very low-certainty evidence). One study reported one infant in the clonidine and morphine group had a seizure, and there were no infant mortalities. The studies did not report neurodevelopmental disability. There may be an increase in days' hospitalisation and days' treatment with clonidine and opioid versus phenobarbital and opioid(hospitalisation: MD 7.13, 95% CI 6.38 to 7.88; treatment: MD 7.57, 95% CI 3.97 to 11.17; both 2 studies, 91 participants; low-certainty evidence). There may be little or no difference in adverse events (RR 1.55, 95% CI 0.44 to 5.40; 2 studies, 93 participants; very low-certainty evidence). However, there was oversedation only in the phenobarbital and morphine group; and hypotension, rebound hypertension and rebound NAS only in the clonidine and morphine group.
Authors' conclusions: There is very low-certainty evidence that phenobarbital increases duration of hospitalisation and treatment, but reduces days to regain birthweight and duration of supportive care each day compared to supportive care alone. There is low-certainty evidence that phenobarbital reduces treatment failure compared to diazepam and very low-certainty evidence that phenobarbital reduces treatment failure compared to chlorpromazine. There is low-certainty evidence of an increase in days' hospitalisation and days' treatment with clonidine and opioid compared to phenobarbital and opioid. There are insufficient data to determine the safety and incidence of adverse events for infants treated with combinations of opioids and sedatives including phenobarbital and clonidine.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
AZ: none.
JM: none.
JGD: none.
DO: none.
Figures










































Update of
-
Sedatives for opiate withdrawal in newborn infants.Cochrane Database Syst Rev. 2010 Oct 6;(10):CD002053. doi: 10.1002/14651858.CD002053.pub3. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2021 May 18;5:CD002053. doi: 10.1002/14651858.CD002053.pub4. PMID: 20927729 Updated.
References
References to studies included in this review
Agthe 2009 {published data only}
-
- Agthe AG, Mathias KB, Hendrix CW, Jansson L, Yaster M, Roark TR, et al. A blinded randomized clinical trial of clonidine in combination with diluted tincture of opium (DTO) versus DTO alone for neonatal abstinence syndrome (NAS). European Journal of Pediatrics 2006;165(S1):11.
-
- Agthe AG, Mathias KB, Hendrix CW, Jansson L, Yaster M, Roark TR, et al. Clonidine in combination with diluted tincture of opium (DTO) versus DTO alone for opioid withdrawal in newborn infants: a blinded randomized clinical trial. In: ePAS. 2007:https://www.pas-meeting.org/past-abstracts/.
-
- Liu T, Lewis T, Gauda E, Gobburu J, Ivaturi V. Mechanistic population pharmacokinetics of morphine in neonates with abstinence syndrome after oral administration of diluted tincture of opium. Journal of Clinical Pharmacology 2016;56(8):1009-18. [DOI: 10.1002/jcph.696] [PMID: 26712409] - DOI - PMC - PubMed
-
- NCT00510016. Clonidine as adjunct therapy for the treatment of neonatal abstinence syndrome (NAS). clinicaltrials.gov/ct2/show/NCT00510016 (first received 1 August 2007).
Brusseau 2020 {published data only}
-
- NCT03670160. Clonidine versus phenobarbital as adjunctive therapy for neonatal abstinence syndrome. clinicaltrials.gov/ct2/show/NCT03670160 (first received 13 September 2018).
Coyle 2002 {published data only}
-
- Bier JB, Ferguson AE, Grenon D, Mullane E, Coyle M. The effects of phenobarbital on developmental outcomes in infants with methadone withdrawal: results of a randomized trial. Pediatric Research 2000;47:175A.
Finnegan 1984a {published and unpublished data}
-
- Finnegan LP, Michael H, Leifer B, Desai S. An evaluation of neonatal abstinence treatment modalities. NIDA Research Monograph 1984;49:282-8. [PMID: ] - PubMed
Kahn 1969 {published data only}
Kaltenbach 1986 {published and unpublished data}
-
- Kaltenbach K, Finnegan LP. Neonatal abstinence syndrome, pharmacotherapy and developmental outcome. Neurobehavioral Toxicology and Teratology 1986;8(4):353-5. [PMID: ] - PubMed
Khoo 1995 {unpublished data only}
-
- Khoo KT. The Effectiveness of Three Treatment Regimens Used in the Management of Neonatal Abstinence Syndrome [PhD thesis]. Melbourne (Australia): University of Melbourne, 1995. [hdl.handle.net/11343/38959]
Madden 1977 {published data only}
Surran 2013 {published data only}
-
- NCT01175668. Clonidine for neonatal abstinence syndrome study [Comparison of clonidine versus phenobarbital as an adjunct therapy for neonatal abstinence syndrome]. clinicaltrials.gov/ct2/show/NCT01175668 (first received 5 August 2010).
-
- Surran B, Visintainer P, Chamberlain S, Kopcza K, Shah B, Singh R. Comparison of clonidine versus phenobarbital as an adjunct therapy for neonatal abstinence syndrome. A prospective randomized clinical trial. Pediatric Academic Societies Annual Meeting; 2013 May 4-7; Washington, DC. 2013. - PubMed
-
- Surran B, Visintainer P, Chamberlain S, Kopcza K, Shah B, Singh R. Efficacy of clonidine versus phenobarbital in reducing neonatal morphine sulfate therapy days for neonatal abstinence syndrome. A prospective randomized clinical trial. Journal of Perinatology 2013;33(12):954-9. [DOI: 10.1038/jp.2013.95] [PMID: ] - DOI - PubMed
Zimmermann 2020 {published data only}
-
- NCT02810782. How to treat opiate withdrawal in neonates [Pharmacological treatment of narcotic neonatal withdrawal]. clinicaltrials.gov/ct2/show/NCT02810782 (first received 23 June 2016).
-
- Zimmermann U, Rudin C, Duo A, Held L, Bucher HU, Swiss neonatal abstinence syndrome study group. Treatment of opioid withdrawal in neonates with morphine, phenobarbital, or chlorpromazine: a randomized double-blind trial. European Journal of Pediatrics 2020;179(1):141-9. [DOI: 10.1007/s00431-019-03486-6] [PMID: ] - DOI - PMC - PubMed
References to studies excluded from this review
Alroomi 1988 {published data only}
Autret 2004 {published data only}
Bada 2015 {published data only}
-
- Bada HS, Sithisarn T, Gibson J, Garlitz K, Caldwell R, Capilouto G, et al. Morphine versus clonidine for neonatal abstinence syndrome. Pediatrics 2015;135(2):e383-91. [DOI: 10.1542/peds.2014-2377] [PMID: ] - PubMed
Calabrese 1985 {published data only}
Carin 1983 {published data only}
Dabek 2013 {published data only}
Daniel 2020 {published data only}
Doberczak 1991 {published data only}
Esmaeili 2010 {published data only}
Finnegan 1975a {published data only}
-
- Finnegan LP, Connaughton JF Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addictive Diseases 1975;2(1-2):141-58. [PMID: ] - PubMed
Finnegan 1975b {published data only}
-
- Finnegan LP, Kron RE, Connaughton JF, Emich JP. Assessment and treatment of abstinence in the infant of the drug-dependent mother. International Journal of Clinical Pharmacology and Biopharmacy 1975;12(1-2):19-32. [PMID: ] - PubMed
Finnegan 1979 {published data only}
-
- Finnegan LP, Mitros TF, Hopkins LE. Management of neonatal narcotic abstinence utilizing a phenobarbital loading dose method. NIDA Research Monograph 1979;27:247-53. [PMID: ] - PubMed
Finnegan 1984b {published data only}
-
- Finnegan LP, Michael H, Leifer B. The use of phenobarbital in treating abstinence in newborns exposed in utero to psychoactive agents. NIDA Research Monograph 1984;49:329. [PMID: ] - PubMed
Harper 1977 {published data only}
Herzlinger 1977 {published data only}
Hoder 1981 {published data only}
Hoder 1984 {published data only}
Kaltenbach 1987 {published data only}
Kandall 1983 {published data only}
Kron 1975a {published data only}
-
- Kron RE, Kaplan SL, Finnegan LP, Litt M, Phoenix MD. The assessment of behavioural change in infants undergoing narcotic withdrawal: comparative data from clinical and objective methods. Addictive Diseases 1975;2(1-2):257-75. [PMID: ] - PubMed
Kron 1975b {published data only}
-
- Kron RE, Litt M, Finnegan LP. Narcotic addiction in the newborn: differences in behaviour generated by methadone and heroin. International Journal of Clinical Pharmacology and Biopharmacy 1975;12(1-2):63-9. - PubMed
Kron 1976 {published data only}
Leikin 2009 {published data only}
Mazurier 2008 {published data only}
Nathenson 1971 {published data only}
-
- Nathenson G, Golden GS, Litt IF. Diazepam in the management of the neonatal narcotic withdrawal syndrome. Pediatrics 1971;48(4):523-7. [PMID: ] - PubMed
NCT01360450 {unpublished data only}
-
- NCT01360450. Clonidine to treat Iatrogenic-induced opioid dependence in infants [Efficacy of clonidine in reducing Iatrogenic-induced opioid dependence in infants]. clinicaltrials.gov/ct2/show/NCT01360450 (first received 25 May 2011).
Ostrea 1975 {published data only}
-
- Ostrea EM, Chavez CJ, Strauss ME. A study of factors that influence the severity of neonatal narcotic withdrawal. Addictive Diseases 1975;2(1-2):187-99. [PMID: ] - PubMed
Ostrea 1976 {published data only}
Pacifico 1989 {published data only}
Sutton 1990 {published data only}
Tunis 1984 {published data only}
-
- Tunis SL, Webster DM, Izes JK, Finnegan LP. Maternal drug use and the effectiveness of pharmacotherapy for neonatal abstinence. NIDA Research Monograph 1984;55:158. [PMID: ] - PubMed
Wolman 1989 {published data only}
Yaster 1996 {published data only}
-
- Yaster M, Kost-Byerly S, Berde C, Billet C. The management of opioid and benzodiazepine dependence in infants, children, and adolescents. Pediatrics 1996;98(1):135-40. [PMID: ] - PubMed
References to ongoing studies
NCT03762317 {published data only}
-
- NCT03762317. Clonidine as adjunct to morphine for neonatal abstinence syndrome [Clonidine as adjunct to morphine in the management of term and near term infants with neonatal abstinence syndrome]. clinicaltrials.gov/ct2/show/NCT03762317 (first received 3 December 2018).
Additional references
AAP 2012
Allegaert 2016
Allocco 2016
Bagley 2014
Bauchinger 2015
Bell 1995
Boucher 2017
Brogly 2014
Brogly 2017
Cleary 2010
Coyle 2012
Dashe 2002
Davies 2016
-
- Davies H, Gilbert R, Johnson K, Petersen I, Nazareth I, O'Donnell M, et al. Neonatal drug withdrawal syndrome: cross country comparison using hospital administrative data in England, the USA, Western Australia and Ontario, Canada. Archives of Disease in Childhood. Fetal and Neonatal Edition 2016;101(1):F26-30. [DOI: 10.1136/archdischild-2015-308948] [PMID: ] - DOI - PubMed
Disher 2019
Finnegan 2005
-
- Finnegan LP, Kandall SR. Maternal and neonatal effects of alcohol and drugs. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors(s). Substance Abuse: a Comprehensive Textbook. Philadelphia (PA): Lippincott Williams & Wilkins, 2005:805-39.
Fricker 1978
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 20 April 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2017. Available at gradepro.org.
Green 1981
Held‐Egli 2009
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Howard 2017
Hulse 1998
Hunt 2008
Jansson 2016
Jones 2010
Jones 2016
Kaltenbach 2012
-
- Kaltenbach K, Holbrook A, Coyle MG, Heil SH, Salisbury A, Stine S, et al. Predicting treatment for neonatal abstinence syndrome in infants born to women maintained on opioid agonist medication. Addiction 2012;107(Suppl 1):45-52. [DOI: 10.1111/j.1360-0443.2012.04038.x] [23106926] - DOI - PMC - PubMed
Kandall 1976
-
- Kandall SR, Albin S, Lowinson J, Berle B, Eidelman AI, Gartner LM. Differential effects of maternal heroin and methadone use on birthweight. Pediatrics 1976;58(5):681-5. [PMID: ] - PubMed
Kandall 1977
Kandall 1993
Kelly 2015
-
- Kelly LE, Knoppert D, Roukema H, Rieder MJ, Koren G. Oral morphine weaning for neonatal abstinence syndrome at home compared with in-hospital: an observational cohort study. Paediatric Drugs 2015;17(2):151-7. [10.1007/s40272-014-0096-y] [PMID: ] - PubMed
Kennare 2005
Kirchner 2014
-
- Kirchner L, Graf-Rohmeister K, Klebermass-Schrehof K, Weninger M, Jagsch R, Metz V, et al. Neonatal abstinence syndrome in European and North American neonates: differences in clinical characteristics derived from a prospective randomised trial. Klinische Pädiatrie 2014;226(5):274-80. [DOI: 10.1055/s-0034-1372586] [PMID: ] - DOI - PubMed
Kocherlakota 2014
Kraft 2012
Kraft 2016
Lam 1992
Lee 2019
Lipsitz 1975
Maas 1990
MacMillan 2018
Marshall 2018
McDonald 2017
-
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane Reviews. Abstracts of the Global Evidence Summit, Cape Town, South Africa. Cochrane Database of Systematic Reviews 2017;9(Suppl 1). [DOI: ]
Middleton 2017
-
- Middleton M, Wright I, Oei JL, Travadi J. Management of infants with neonatal abstinence syndrome in a tertiary care hospital. Journal of Paediatrics and Child Health 2017;53(Suppl 2):106.
Mizrahi 1987
NDSHS 2013
-
- Australian Institute of Health and Welfare. National Drug Strategy Household Survey detailed report 2013. www.aihw.gov.au/reports/illicit-use-of-drugs/ndshs-2013-detailed/content... (accessed 28 January 2021).
Newman 2015
NHSDA 1999
-
- Office of Applied Statistics, Substance Abuse and Mental Health Administration (SAMHSA). National household survey on drug abuse. 1999. www.datafiles.samhsa.gov/study/national-household-survey-drug-abuse-nhsd... (accessed 28 January 2021).
NHSDA 2013
-
- Substance Abuse and Mental Health Administration (SAMHSA). National household survey on drug abuse. 2013. samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHre... (accessed 28 January 2021).
Noel‐Storr 2018
-
- Noel-Storr AH and the Project Transform team. Cochrane Crowd and Classmate: new ways of working and learning together to produce health evidence. Abstracts of the 26th Cochrane Colloquium, Santiago, Chile. Cochrane Database of Systematic Reviews 2020;1(Suppl 1). [DOI: ]
Oei 2017
Olofsson 1983
Orlando 2014
Oro 1988
-
- Oro AS, Dixon SD. Waterbed care of narcotic-exposed neonates. A useful adjunct to supportive care. American Journal of Diseases of Children 1988;142:186-8. - PubMed
Pahl 2020
Patel 2013
Patrick 2015
Peltz 2015
Raith 2015
-
- Raith W, Schmölzer BR, Fritz Reiterer A. Laser acupuncture for neonatal abstinence syndrome: a randomised controlled trial. Pediatrics 2015;136(5):876-84. - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Short 2016
Siu 2014
Spiteri Cornish 2013
-
- Spiteri Cornish K, Hrabovsky M, Scott NW, Myerscough E, Reddy AR. The short and long-term, effects on the visual system of children following exposure to maternal substance misuse in pregnancy. American Journal of Ophthalmology 2013;156(1):190-4. [DOI: 10.1016/j.ajo.2013.02.004] [PMID: ] - DOI - PubMed
Stover 2015
Strauss 1976
Sundelin Wahlsten 2013
Theis 1997
Thomas 2017
Tolia 2015
Turner 2015
Unger 2012
Visconti 2013
-
- Visconti K, Hennessy K, Towers C, Hannessy M, Howard B. Opiate use in pregnancy and newborn head circumference. American Journal of Obstetrics and Gynecology 2013;208:S67-8.
Volpe 2008
-
- Volpe JJ. Neonatal seizures. In: Neurology of the Newborn. Philadelphia (PA): Saunders Elsevier, 2008:203-44. [ISBN: 9781416039952]
Wachman 2018
Walhovd 2007
Walhovd 2012
WHO 2014
-
- World Health Organization. Guidelines for the identification and management of substance use and substance use disorders in pregnancy. who.int/iris/bitstream/10665/107130/1/9789241548731_eng.pdf (accessed April 2017). - PubMed
Wolff 2014
Zahorodny 1998
References to other published versions of this review
Osborn 2000
Osborn 2002
Osborn 2005
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

