Evaluation of six different rapid methods for nucleic acid detection of SARS-COV-2 virus
- PMID: 34002401
- PMCID: PMC8242416
- DOI: 10.1002/jmv.27090
Evaluation of six different rapid methods for nucleic acid detection of SARS-COV-2 virus
Abstract
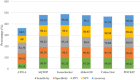
In the current coronavirus disease 2019 (COVID-19) pandemic there is a mass screening of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) happening around the world due to the extensive spread of the infections. There is a high demand for rapid diagnostic tests to expedite the identification of cases and to facilitate early isolation and control spread. Hence this study evaluates six different rapid nucleic acid detection assays that are commercially available for SARS-CoV-2 virus detection. Nasopharyngeal samples were collected from 4981 participants and were tested for the SARS-CoV-2 virus by the gold standard real-time reverse-transcription polymerase chain reaction (RT-PCR) method and with one of these six rapid methods of detection. Evaluation of the rapid nucleic acid detection assays was done by comparing the results of these rapid methods with the gold standard RT-qPCR results for SARS-COV-2 detection. AQ-TOP had the highest sensitivity (98%) and a strong kappa value of 0.943 followed by Genechecker and Abbot ID NOW. The POCKIT (ii RT-PCR) assay had the highest test accuracy of 99.29% followed by Genechecker and Cobas Liat. Atila iAMP showed the highest percentage of invalid reports (35.5%) followed by AQ-TOP with 6% and POCKIT with 3.7% of invalid reports. Genechecker system, Abbott ID NOW, and Cobas Liat were found to have the best performance and agreement when compared with the standard RT-PCR for COVID-19 detection. With further research, these rapid tests have the potential to be employed in large-scale screening of COVID-19.
Keywords: COVID-19; SARS-CoV-2; nucleic acid tests; rapid tests.
© 2021 G42 Healthcare. Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures
Similar articles
-
Multisite Clinical Validation of Isothermal Amplification-Based SARS-CoV-2 Detection Assays Using Different Sampling Strategies.Microbiol Spectr. 2021 Oct 31;9(2):e0084621. doi: 10.1128/Spectrum.00846-21. Epub 2021 Oct 20. Microbiol Spectr. 2021. PMID: 34668736 Free PMC article.
-
Clinical Performance of the Point-of-Care cobas Liat for Detection of SARS-CoV-2 in 20 Minutes: a Multicenter Study.J Clin Microbiol. 2021 Jan 21;59(2):e02811-20. doi: 10.1128/JCM.02811-20. Print 2021 Jan 21. J Clin Microbiol. 2021. PMID: 33239382 Free PMC article.
-
Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand.Virol J. 2020 Nov 13;17(1):177. doi: 10.1186/s12985-020-01452-5. Virol J. 2020. PMID: 33187528 Free PMC article.
-
Clinical performance of the Roche Cobas Liat SARS-CoV-2 & influenza A/B assay: A systematic review and meta-analysis.J Clin Virol. 2024 Oct;174:105706. doi: 10.1016/j.jcv.2024.105706. Epub 2024 Jun 7. J Clin Virol. 2024. PMID: 38908267
-
Ravaging SARS-CoV-2: rudimentary diagnosis and puzzling immunological responses.Curr Med Res Opin. 2021 Feb;37(2):207-217. doi: 10.1080/03007995.2020.1862532. Epub 2020 Dec 26. Curr Med Res Opin. 2021. PMID: 33306409 Free PMC article. Review.
Cited by
-
SARS-CoV-2 Testing of Emergency Department Patients Using cobas® Liat® and eazyplex® Rapid Molecular Assays.Diagnostics (Basel). 2023 Jul 1;13(13):2245. doi: 10.3390/diagnostics13132245. Diagnostics (Basel). 2023. PMID: 37443639 Free PMC article.
-
[Implementation of isotermic PCR for detection of SARS-CoV-2 virus].Rev Med Inst Mex Seguro Soc. 2024 May 6;62(3):1-6. doi: 10.5281/zenodo.10998931. Rev Med Inst Mex Seguro Soc. 2024. PMID: 39530769 Free PMC article. Spanish.
-
Evaluation of Four Fully Integrated Molecular Assays for the Detection of Respiratory Viruses during the Co-Circulation of SARS-CoV-2, Influenza and RSV.J Clin Med. 2022 Jul 6;11(14):3942. doi: 10.3390/jcm11143942. J Clin Med. 2022. PMID: 35887705 Free PMC article.
-
A Colorimetric RT-LAMP Assay for Rapid Detection of Soybean mosaic Virus SC15.ACS Omega. 2024 Jun 24;9(27):29765-29775. doi: 10.1021/acsomega.4c03372. eCollection 2024 Jul 9. ACS Omega. 2024. PMID: 39005798 Free PMC article.
-
Performance comparison of two nucleic acid amplification systems for SARS-CoV-2 detection: A multi-center study.J Clin Lab Anal. 2022 Nov;36(11):e24727. doi: 10.1002/jcla.24727. Epub 2022 Oct 4. J Clin Lab Anal. 2022. PMID: 36196490 Free PMC article.
References
-
- Worldometers . 2020. https://www.worldometers.info/coronavirus/country/. Accessed February 23, 2021.
-
- World Health Organization . Laboratory testing strategy recommendations for COVID‐19. Interim guidance. 2020. https://apps.who.int/iris/bitstream/handle/10665/331509/WHO-COVID-19-lab.... Accessed December 12, 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous