Assessment of interferon-γ in pleural fluid as a prognostic factor of survival in malignant pleural mesothelioma
- PMID: 34003301
- PMCID: PMC10992072
- DOI: 10.1007/s00262-021-02965-w
Assessment of interferon-γ in pleural fluid as a prognostic factor of survival in malignant pleural mesothelioma
Abstract
Backgound: Literature reports suggest that the host immune system may control Malignant Pleural Mesothelioma (MPM) growth, although its activity is limited by regulatory mechanisms. In this retrospective study, we analyzed the levels of pro-inflammatory (IL-1, IL-6, TNF), immune-regulatory (IL-10) and Th1/CTL-related cytokines (IL-12p70, IFN-γ) in the pleural exudate and their relationship with overall survival (OS) in MPM.
Methods: Cytokines were quantified by multiplexed immunoassay. Concentrations were dichotomized with respect to the median value. Correlation between cytokine level and OS was assessed using univariate (Kaplan-Meier curves) and multivariate (Cox regression) analyses.
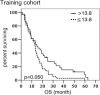
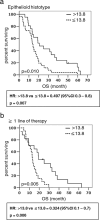
Results: Regarding outcome, tumor histology, therapies undergone and IFN-γ were independent prognostic factors of OS in a 72 MPM training cohort. Notably, high concentrations of IFN-γ halved death probability (HR of high vs low IFN-γ concentration = 0.491, 95%CI 0.3-0.8, p = 0.007). Also in patients with epithelioid histology and those receiving at least one line of therapy, high IFN-γ level was an independent factor predictive of OS (HR of high vs low IFN-γ concentration were 0.497, p = 0.007 and 0.324, p = 0.006, respectively). However, these data were not confirmed in a 77 MPM validation cohort, possibly due to the low IFN-γ levels encountered in this population, and the heterogeneous distribution of disease stages between the training and the validation cohorts. None of the other cytokines showed any effect on survival.
Conclusions: High level of IFN-γ in pleural effusion may be associated with better survival in MPM patients and potentially serve as a prognostic biomarker. Larger prospective studies are needed to ascertain this hypothesis.
Keywords: Biomarker; Immune response; Interferon-gamma; Mesothelioma; Outcome; Pleural effusion.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The author declares there is no conflict of interest.
Figures

References
-
- Maio M, Scherpereel A, Calabrò L, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017;18:1261–1273. doi: 10.1016/S1470-2045(17)30446-1. - DOI - PubMed
-
- Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20:239–253. doi: 10.1016/S1470-2045(18)30765-4. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

