Hepatocyte HIF-1 and Intermittent Hypoxia Independently Impact Liver Fibrosis in Murine Nonalcoholic Fatty Liver Disease
- PMID: 34003729
- PMCID: PMC8525205
- DOI: 10.1165/rcmb.2020-0492OC
Hepatocyte HIF-1 and Intermittent Hypoxia Independently Impact Liver Fibrosis in Murine Nonalcoholic Fatty Liver Disease
Abstract
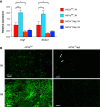
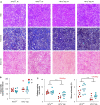
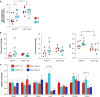
Obstructive sleep apnea is associated with insulin resistance, lipid dysregulation, and hepatic steatosis and fibrosis in nonalcoholic fatty liver disease (NAFLD). We have previously shown that hepatocyte HIF-1 (hypoxia-inducible factor-1) mediates the development of liver fibrosis in a mouse model of NAFLD. We hypothesized that intermittent hypoxia (IH) modeling obstructive sleep apnea would worsen hepatic steatosis and fibrosis in murine NAFLD, via HIF-1. Mice with hepatocyte-specific deletion of Hif1a (Hif1a-/-hep) and wild-type (Hif1aF/F) controls were fed a high trans-fat diet to induce NAFLD with steatohepatitis. Half from each group were exposed to IH, and the other half were exposed to intermittent air. A glucose tolerance test was performed just prior to the end of the experiment. Mitochondrial efficiency was assessed in fresh liver tissue at the time of death. The hepatic malondialdehyde concentration and proinflammatory cytokine levels were assessed, and genes of collagen and fatty acid metabolism were examined. Hif1a-/-hep mice gained less weight than wild-type Hif1a mice (-2.3 g, P = 0.029). There was also a genotype-independent effect of IH on body weight, with less weight gain in mice exposed to IH (P = 0.003). Fasting glucose, homeostatic model assessment for insulin resistance, and glucose tolerance test results were all improved in Hif1a-/-hep mice. Liver collagen was increased in mice exposed to IH (P = 0.033) and was reduced in Hif1a-/-hep mice (P < 0.001), without any significant exposure/genotype interaction being demonstrated. Liver TNF-α and IL-1β were significantly increased in mice exposed to IH and were decreased in Hif1a-/-hep mice. We conclude that HIF-1 signaling worsens the metabolic profile and hastens NAFLD progression and that IH may worsen liver fibrosis. These effects are plausibly mediated by hepatic inflammatory stress.
Keywords: HIF-1; hepatic fibrosis; hyperglycemia; lipid metabolism; obstructive sleep apnea.
Figures


Comment in
-
Sleep Apnea, Hypoxia Inducible Factor, and Fatty Liver: More Questions Than Answers?Am J Respir Cell Mol Biol. 2021 Oct;65(4):337-338. doi: 10.1165/rcmb.2021-0204ED. Am J Respir Cell Mol Biol. 2021. PMID: 34101539 Free PMC article. No abstract available.
References
-
- Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC. et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

