Integrated sensing of host stresses by inhibition of a cytoplasmic two-component system controls M. tuberculosis acute lung infection
- PMID: 34003742
- PMCID: PMC8131098
- DOI: 10.7554/eLife.65351
Integrated sensing of host stresses by inhibition of a cytoplasmic two-component system controls M. tuberculosis acute lung infection
Abstract
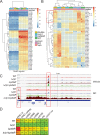
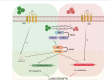
Bacterial pathogens that infect phagocytic cells must deploy mechanisms that sense and neutralize host microbicidal effectors. For Mycobacterium tuberculosis, the causative agent of tuberculosis, these mechanisms allow the bacterium to rapidly adapt from aerosol transmission to initial growth in the lung alveolar macrophage. Here, we identify a branched signaling circuit in M. tuberculosis that controls growth in the lung through integrated direct sensing of copper ions and nitric oxide by coupled activity of the Rip1 intramembrane protease and the PdtaS/R two-component system. This circuit uses a two-signal mechanism to inactivate the PdtaS/PdtaR two-component system, which constitutively represses virulence gene expression. Cu and NO inhibit the PdtaS sensor kinase through a dicysteine motif in the N-terminal GAF domain. The NO arm of the pathway is further controlled by sequestration of the PdtaR RNA binding response regulator by an NO-induced small RNA, controlled by the Rip1 intramembrane protease. This coupled Rip1/PdtaS/PdtaR circuit controls NO resistance and acute lung infection in mice by relieving PdtaS/R-mediated repression of isonitrile chalkophore biosynthesis. These studies identify an integrated mechanism by which M. tuberculosis senses and resists macrophage chemical effectors to achieve pathogenesis.
Keywords: M. tuberculosis; bacterial pathogenesis; infectious disease; microbiology; nutritional immunity; signal transduction; tuberculosis.
© 2021, Buglino et al.
Conflict of interest statement
JB, GS, NL, JB No competing interests declared, MG MSG is a consultant for Fimbrion Therapeutics, serves on the SAB of Vedanta Biosciences and receives consulting fees and equity, and serves of the SAB of PRL NYC.
Figures

References
-
- Bhatt K, Machado H, Osório NS, Sousa J, Cardoso F, Magalhães C, Chen B, Chen M, Kim J, Singh A, Ferreira CM, Castro AG, Torrado E, Jacobs WR, Bhatt A, Saraiva M. A nonribosomal peptide synthase gene driving virulence in Mycobacterium tuberculosis. mSphere. 2018;3:e00352-18. doi: 10.1128/mSphere.00352-18. - DOI - PMC - PubMed
-
- Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charrière GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host & Microbe. 2011;10:248–259. doi: 10.1016/j.chom.2011.08.006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

