Longitudinal Serology of SARS-CoV-2-Infected Individuals in India: A Prospective Cohort Study
- PMID: 34003792
- PMCID: PMC8274753
- DOI: 10.4269/ajtmh.21-0164
Longitudinal Serology of SARS-CoV-2-Infected Individuals in India: A Prospective Cohort Study
Abstract
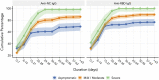
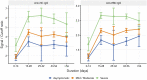
Clinical and epidemiological characteristics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are now widely available, but there are few data regarding longitudinal serology in large cohorts, particularly those from low-income and middle-income countries. We established an ongoing prospective cohort of 3,840 SARS-CoV-2-positive individuals according to RT-PCR in the Delhi-National Capital Region of India to document clinical and immunological characteristics during illness and convalescence. The immunoglobulin G (IgG) responses to the receptor binding domain (RBD) and nucleocapsid were assessed at 0 to 7 days, 10 to 28 days, and 6 to 10 weeks after infection. The clinical predictors of seroconversion were identified by multivariable regression analysis. The seroconversion rates during the postinfection windows of 0 to 7 days, 10 to 28 days, and 6 to 10 weeks were 46%, 84.7%, and 85.3%, respectively (N = 743). The proportion with a serological response increased with the severity of coronavirus disease 2019 (COVID-19). All participants with severe disease, 89.6% with mild to moderate infection, and 77.3% of asymptomatic participants had IgG antibodies to the RBD antigen. The threshold values for the nasopharyngeal viral RNA RT-PCR of a subset of asymptomatic and symptomatic seroconverters were comparable (P = 0.48) to those of nonseroconverters (P = 0.16) (N = 169). This is the first report of longitudinal humoral immune responses to SARS-CoV-2 over a period of 10 weeks in South Asia. The low seropositivity of asymptomatic participants and differences between assays highlight the importance of contextualizing the understanding of population serosurveys.
Figures
References
-
- Indian Council of Medical Research , 2020. Testing Strategy. Available at: https://www.icmr.gov.in/cteststrat.html.
-
- Ministry of Health and Family Welfare, Government of India, 2019. Sample collection packaging. Available at: https://www.mohfw.gov.in/pdf/5Sample%20collection_packaging%20%202019-nC....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

