Real-life implementation of a G6PD deficiency screening qualitative test into routine vivax malaria diagnostic units in the Brazilian Amazon (SAFEPRIM study)
- PMID: 34003840
- PMCID: PMC8162658
- DOI: 10.1371/journal.pntd.0009415
Real-life implementation of a G6PD deficiency screening qualitative test into routine vivax malaria diagnostic units in the Brazilian Amazon (SAFEPRIM study)
Abstract
Background: Glucose-6-phosphate dehydrogenase (G6PD) deficiency greatly hinders Plasmodium vivax malaria radical cure and further elimination due to 8-aminoquinolines-associated hemolysis. Although the deleterious health effects of primaquine in G6PD deficient individuals have been known for over 50 years, G6PD testing is not routinely performed before primaquine treatment in most P. vivax endemic areas.
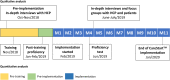
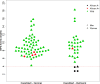
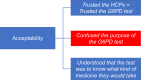
Method/principal findings: The qualitative CareStart G6PD screening test was implemented in 12 malaria treatment units (MTUs) in the municipality of Rio Preto da Eva, Western Brazilian Amazon, a malaria endemic area, between February 2019 and early January 2020. Training materials were developed and validated; evaluations were conducted on the effectiveness of training health care professionals (HCPs) to perform the test, the interpretation and reliability of routine testing performed by HCPs, and perceptions of HCPs and patients. Most HCPs were unaware of G6PD deficiency and primaquine-related adverse effects. Most of 110 HCPs trained (86/110, 78%) were able to correctly perform the G6PD test after a single 4-hour training session. The test performed by HCPs during implementation showed 100.0% (4/4) sensitivity and 68.1% (62/91) specificity in identifying G6PD deficient patients as compared to a point-of-care quantitative test (Standard G6PD).
Conclusions/significance: G6PD screening using the qualitative CareStart G6PD test performed by HCPs in MTUs of an endemic area showed high sensitivity and concerning low specificity. The amount of false G6PD deficiency detected led to substantial loss of opportunities for radical cure.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Quantitative G6PD Deficiency Screening in Routine Malaria Diagnostic Units in the Brazilian Amazon (SAFEPRIM): An Operational Mixed-Methods Study.Pathogens. 2022 Nov 11;11(11):1328. doi: 10.3390/pathogens11111328. Pathogens. 2022. PMID: 36422580 Free PMC article.
-
Perspectives of healthcare professionals on training for quantitative G6PD testing during implementation of tafenoquine in Brazil (QualiTRuST Study).PLoS Negl Trop Dis. 2024 Jun 5;18(6):e0012197. doi: 10.1371/journal.pntd.0012197. eCollection 2024 Jun. PLoS Negl Trop Dis. 2024. PMID: 38837977 Free PMC article.
-
Global economic costs due to vivax malaria and the potential impact of its radical cure: A modelling study.PLoS Med. 2021 Jun 1;18(6):e1003614. doi: 10.1371/journal.pmed.1003614. eCollection 2021 Jun. PLoS Med. 2021. PMID: 34061843 Free PMC article.
-
Use of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: Divergent policies and practices in malaria endemic countries.PLoS Negl Trop Dis. 2018 Apr 19;12(4):e0006230. doi: 10.1371/journal.pntd.0006230. eCollection 2018 Apr. PLoS Negl Trop Dis. 2018. PMID: 29672516 Free PMC article. Review.
-
Primaquine alternative dosing schedules for preventing malaria relapse in people with Plasmodium vivax.Cochrane Database Syst Rev. 2020 Aug 19;8:CD012656. doi: 10.1002/14651858.CD012656.pub3. Cochrane Database Syst Rev. 2020. PMID: 32816320
Cited by
-
Prevalence of glucose 6-phosphate dehydrogenase deficiency in highly malaria-endemic municipalities in the Brazilian Amazon: A region-wide screening study.Lancet Reg Health Am. 2022 May 26;12:100273. doi: 10.1016/j.lana.2022.100273. eCollection 2022 Aug. Lancet Reg Health Am. 2022. PMID: 36776424 Free PMC article.
-
Contextual factors and G6PD diagnostic testing: a scoping review and evidence and gap map.Malar J. 2024 Aug 12;23(1):241. doi: 10.1186/s12936-024-05050-6. Malar J. 2024. PMID: 39135005 Free PMC article.
-
Safety and Efficacy of Tafenoquine for Plasmodium vivax Malaria Prophylaxis and Radical Cure: Overview and Perspectives.Ther Clin Risk Manag. 2021 Sep 8;17:989-999. doi: 10.2147/TCRM.S269336. eCollection 2021. Ther Clin Risk Manag. 2021. PMID: 34526770 Free PMC article.
-
Accelerating towards P. vivax elimination with a novel serological test-and-treat strategy: a modelling case study in Brazil.Lancet Reg Health Am. 2023 May 19;22:100511. doi: 10.1016/j.lana.2023.100511. eCollection 2023 Jun. Lancet Reg Health Am. 2023. PMID: 37250687 Free PMC article.
-
CUREMA project: a further step towards malaria elimination among hard-to-reach and mobile populations.Malar J. 2024 Sep 10;23(1):271. doi: 10.1186/s12936-024-05040-8. Malar J. 2024. PMID: 39256842 Free PMC article.
References
-
- Brito-Sousa JD, Santos TC, Avalos S, Fontecha G, Melo GC, Val F, et al.. Clinical Spectrum of Primaquine-induced Hemolysis in Glucose-6-Phosphate Dehydrogenase Deficiency: A 9-Year Hospitalization-based Study From the Brazilian Amazon. Clin Infect Dis. 2019;69:1440–1442. 10.1093/cid/ciz122 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

