Nitric oxide coordinates growth, development, and stress response via histone modification and gene expression
- PMID: 34003928
- PMCID: PMC8418403
- DOI: 10.1093/plphys/kiab222
Nitric oxide coordinates growth, development, and stress response via histone modification and gene expression
Abstract
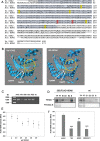
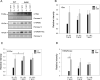
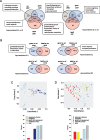
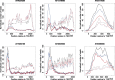
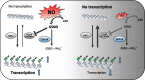
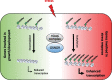
Nitric oxide (NO) is a signaling molecule with multiple regulatory functions in plant physiology and stress response. In addition to direct effects on transcriptional machinery, NO executes its signaling function via epigenetic mechanisms. We report that light intensity-dependent changes in NO correspond to changes in global histone acetylation (H3, H3K9, and H3K9/K14) in Arabidopsis (Arabidopsis thaliana) wild-type leaves, and that this relationship depends on S-nitrosoglutathione reductase and histone deacetylase 6 (HDA6). The activity of HDA6 was sensitive to NO, demonstrating that NO participates in regulation of histone acetylation. Chromatin immunoprecipitation sequencing and RNA-seq analyses revealed that NO participates in the metabolic switch from growth and development to stress response. This coordinating function of NO might be particularly important in plant ability to adapt to a changing environment, and is therefore a promising foundation for mitigating the negative effects of climate change on plant productivity.
© The Author(s) 2021. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Figures




References
-
- An L, Liu Y, Zhang M, Chen T, Wang X (2005) Effects of nitric oxide on growth of maize seedling leaves in the presence or absence of ultraviolet-B radiation. J Plant Physiol 162: 317–326 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

