Clinical 3D Imaging of the Anterior Segment With Ultrasound Biomicroscopy
- PMID: 34003945
- PMCID: PMC7961115
- DOI: 10.1167/tvst.10.3.11
Clinical 3D Imaging of the Anterior Segment With Ultrasound Biomicroscopy
Abstract
Purpose: Ultrasound biomicroscopy (UBM) is an important ophthalmic imaging modality due to its ability to see behind pigmented iris and to visualize anterior chamber when the eye's transparency is compromised. We created a three-dimensional UBM (3D-UBM) system and acquired example images to illustrate its potential.
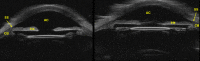
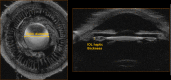
Methods: A commercial 50-MHz two-dimensional UBM (2D-UBM) system was attached to a precision translation stage and translated across the eye to acquire an image volume. The stage was mounted on a surgical microscope, which enabled safe, stable positioning. Image processing steps included image alignment, noise reduction, and calibration. 3D visualization included alignment of the optic axis, multiplanar reformatting at arbitrary orientations, and volume rendering with optimized transfer functions. Scans were performed on cadaver and rabbit eyes.
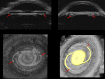
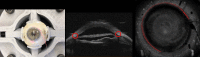
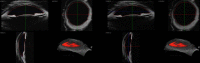
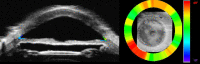
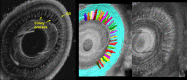
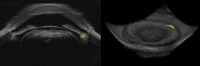
Results: 3D-UBM allowed visualization of the anterior segment tissues within a 3D anatomical context, unlike 2D-UBM. En face views and interactive slicer operations suggested an ability to plan and assess treatments, including lens placement and microcatheter cannulation of Schlemm's canal. Interactive software allowed us to make accurate measurements of tissue structures (e.g., iridocorneal angles, cyst volumes). In addition, unique measurements of ciliary tissues included single ciliary process volumes of 0.234 ± 0.093 mm3 with surface areas of 3.02 ± 1.07 mm2 and ciliary muscle volume of 67.87 mm3.
Conclusions: 3D-UBM imaging of the anterior segment can be used to enable unique visualization and quantification of anterior segment structures.
Translational relevance: 3D-UBM provides informative 3D imaging of tissues in the eye that are invisible to light to potentially provide physicians with improved diagnosis, treatment planning, and treatment assessment as compared to conventional 2D-UBM.
Conflict of interest statement
Disclosure:
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

