Outpatient Management of Patients With COVID-19: Multicenter Prospective Validation of the Hospitalization or Outpatient Management of Patients With SARS-CoV-2 Infection Rule to Discharge Patients Safely
- PMID: 34004154
- PMCID: PMC8123410
- DOI: 10.1016/j.chest.2021.05.008
Outpatient Management of Patients With COVID-19: Multicenter Prospective Validation of the Hospitalization or Outpatient Management of Patients With SARS-CoV-2 Infection Rule to Discharge Patients Safely
Abstract
Background: The Hospitalization or Outpatient Management of Patients With SARS-CoV-2 Infection (HOME-CoV) rule is a checklist of eligibility criteria for home treatment of patients with COVID-19, defined using a Delphi method.
Research question: Is the HOME-CoV rule reliable for identifying a subgroup of COVID-19 patients with a low risk of adverse outcomes who can be treated at home safely?
Study design and methods: We aimed to validate the HOME-CoV rule in a prospective, multicenter study before and after trial of patients with probable or confirmed COVID-19 who sought treatment at the ED of 34 hospitals. The main outcome was an adverse evolution, that is, invasive ventilation or death, occurring within the 7 days after patient admission. The performance of the rule was assessed by the false-negative rate. The impact of the rule implementation was assessed by the absolute differences in the rate of patients who required invasive ventilation or who died and in the rate of patients treated at home, between an observational and an interventional period after implementation of the HOME-CoV rule, with propensity score adjustment.
Results: Among 3,000 prospectively enrolled patients, 1,239 (41.3%) demonstrated a negative HOME-CoV rule finding. The false-negative rate of the HOME-CoV rule was 4 in 1,239 (0.32%; 95% CI, 0.13%-0.84%), and its area under the receiver operating characteristic curve was 80.9 (95% CI, 76.5-85.2). On the adjusted populations, 25 of 1,274 patients (1.95%) experienced an adverse evolution during the observational period vs 12 of 1,274 patients (0.95%) during the interventional period: -1.00 (95% CI, -1.86 to -0.15). During the observational period, 858 patients (67.35%) were treated at home vs 871 patients (68.37%) during the interventional period: -1.02 (95% CI, -4.46 to 2.26).
Interpretation: A large proportion of patients treated in the ED with probable or confirmed COVID-19 have a negative HOME-CoV rule finding and can be treated safely at home with a very low risk of complications.
Trial registry: ClinicalTrials.gov; No.: NCT04338841; URL: www.clinicaltrials.gov.
Keywords: COVID-19; clinical support decision tool; expert consensus; hospitalization; outpatient; rule validation; rule-based decision-making.
Copyright © 2021 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures
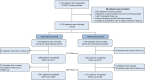
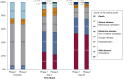
Comment in
-
Hospital or Home?: A Pandemic Decision Tool in Context.Chest. 2021 Oct;160(4):1155-1156. doi: 10.1016/j.chest.2021.06.024. Chest. 2021. PMID: 34625158 Free PMC article. No abstract available.
References
-
- Zhang H, Shi T, Wu X, et al. Risk prediction for poor outcome and death in hospital in-patients with COVID-19: derivation in Wuhan, China and external validation in London, UK [published online ahead of print May 3, 2020]. MedRxiv. 2020:2020.04.28.20082222.
-
- Song C-Y, Xu J, He J-Q, Lu Y-Q. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients [published online ahead of print March 8, 2020]. MedRxiv. https://doi.org/10.1101/2020.03.05.20031906. - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

