Test-based de-isolation in COVID-19 immunocompromised patients: Cycle threshold value versus SARS-CoV-2 viral culture
- PMID: 34004329
- PMCID: PMC8123529
- DOI: 10.1016/j.ijid.2021.05.027
Test-based de-isolation in COVID-19 immunocompromised patients: Cycle threshold value versus SARS-CoV-2 viral culture
Abstract
Background: Immunocompromised patients with coronavirus disease 2019 (COVID-19) have prolonged infectious viral shedding for more than 20 days. A test-based approach is suggested for de-isolation of these patients.
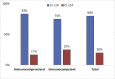
Methods: The strategy was evaluated by comparing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load (cycle threshold (Ct) values) and viral culture at the time of hospital discharge in a series of 13 COVID-19 patients: six immunocompetent and seven immunocompromised (five solid organ transplant patients, one lymphoma patient, and one hepatocellular carcinoma patient).
Results: Three of the 13 (23%) patients had positive viral cultures: one patient with lymphoma (on day 16) and two immunocompetent patients (on day 7 and day 11). Eighty percent of the patients had negative viral cultures and had a mean Ct value of 20.5. None of the solid organ transplant recipients had positive viral cultures.
Conclusions: The mean Ct value for negative viral cultures was 20.5 in this case series of immunocompromised patients. Unlike those with hematological malignancies, none of the solid organ transplant patients had positive viral cultures. Adopting the test-based approach for all immunocompromised patients may lead to prolonged quarantine. Large-scale studies in disease-specific populations are needed to determine whether a test-based approach versus a symptom-based approach or a combination is applicable for the de-isolation of various immunocompromised patients.
Keywords: Immunocompromised patients; Isolation; SARS-CoV-2; Viral culture.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
Similar articles
-
Predicting persistent SARS-CoV-2 shedding in immunocompromised patients: a probability-based approach.Infect Dis (Lond). 2025 May;57(5):407-416. doi: 10.1080/23744235.2024.2446286. Epub 2025 Jan 3. Infect Dis (Lond). 2025. PMID: 39749573
-
Isolation of SARS-CoV-2 in Viral Cell Culture in Immunocompromised Patients With Persistently Positive RT-PCR Results.Front Cell Infect Microbiol. 2022 Feb 2;12:804175. doi: 10.3389/fcimb.2022.804175. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35186791 Free PMC article.
-
Prolonged viral shedding of SARS-CoV-2 in two immunocompromised patients, a case report.BMC Infect Dis. 2021 Aug 3;21(1):743. doi: 10.1186/s12879-021-06429-5. BMC Infect Dis. 2021. PMID: 34344321 Free PMC article.
-
Viral cultures, cycle threshold values and viral load estimation for assessing SARS-CoV-2 infectiousness in haematopoietic stem cell and solid organ transplant patients: a systematic review.J Hosp Infect. 2023 Feb;132:62-72. doi: 10.1016/j.jhin.2022.11.018. Epub 2022 Dec 5. J Hosp Infect. 2023. PMID: 36473552 Free PMC article.
-
A Systematic Review of Prolonged SARS-CoV-2 Shedding in Immunocompromised Persons.Influenza Other Respir Viruses. 2025 May;19(5):e70121. doi: 10.1111/irv.70121. Influenza Other Respir Viruses. 2025. PMID: 40394759 Free PMC article.
Cited by
-
Liver resection in a patient with persistent positive PCR test for coronavirus disease 2019 (COVID-19): a case report.Surg Case Rep. 2022 Oct 20;8(1):200. doi: 10.1186/s40792-022-01553-z. Surg Case Rep. 2022. PMID: 36264514 Free PMC article.
-
Duration of viable SARS-CoV-2 shedding from respiratory tract in different human hosts and its impact on isolation discontinuation polices revision; a narrative review.Clin Infect Pract. 2022 Jan;13:100140. doi: 10.1016/j.clinpr.2022.100140. Epub 2022 Feb 16. Clin Infect Pract. 2022. PMID: 35190799 Free PMC article. Review.
-
In silico prediction and experimental evaluation of potential siRNAs against SARS-CoV-2 inhibition in Vero E6 cells.J King Saud Univ Sci. 2022 Jun;34(4):102049. doi: 10.1016/j.jksus.2022.102049. Epub 2022 Apr 26. J King Saud Univ Sci. 2022. PMID: 35493709 Free PMC article.
-
COVID-19 isolation strategies: What have we learned.Travel Med Infect Dis. 2022 Sep-Oct;49:102416. doi: 10.1016/j.tmaid.2022.102416. Epub 2022 Aug 8. Travel Med Infect Dis. 2022. PMID: 35952965 Free PMC article.
-
Duration of SARS-CoV-2 shedding and infectivity in the working age population: a systematic review and meta-analysis.Med Lav. 2022 Apr 26;113(2):e2022014. doi: 10.23749/mdl.v113i2.12724. Med Lav. 2022. PMID: 35481581 Free PMC article.
References
-
- Azhar E.I., Hindawi S.I., El-Kafrawy S.A., Hassan A.M., Tolah A.M., Alandijany T.A. Amotosalen and ultraviolet A light treatment efficiently inactivates severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in human plasma. Vox Sanguinis. 2020;(December) doi: 10.1111/vox.13043. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous