Targeting SERT promotes tryptophan metabolism: mechanisms and implications in colon cancer treatment
- PMID: 34006301
- PMCID: PMC8132442
- DOI: 10.1186/s13046-021-01971-1
Targeting SERT promotes tryptophan metabolism: mechanisms and implications in colon cancer treatment
Abstract
Background: Serotonin signaling has been associated with tumorigenesis and tumor progression. Targeting the serotonin transporter to block serotonin cellular uptake confers antineoplastic effects in various tumors, including colon cancer. However, the antineoplastic mechanism of serotonin transporter inhibition and serotonin metabolism alterations in the absence of serotonin transporter have not been elucidated, especially in colon cancer, which might limit anti-tumor effects associating with targeting serotonin transporter.
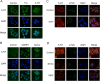
Methods: The promotion in the uptake and catabolism of extracellular tryptophan and targeting serotonin transporter was detected by using quantitative reverse-transcription polymerase chain reaction, western blotting and liquid chromatography tandem mass spectrometry. Western blotting Immunoprecipitation and immunofluorescence was utilized to research the serotonylation of mTOR by serotonin and serotonin transporter inhibition. The primary mouse model, homograft model and tissue microarry was used to explore the tryptophan pathway in colon cancer. The cell viability assay, western blotting, xenograft and primary colon cancer mouse model were used to identify whether the combination of sertraline and tryptophan restriction had a synergistic effect.
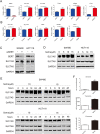
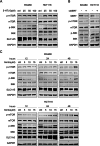
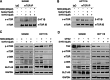
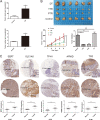
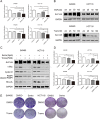
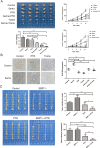
Results: Targeting serotonin transporter through genetic ablation or pharmacological inhibition in vitro and in vivo induced a compensatory effect by promoting the uptake and catabolism of extracellular tryptophan in colon cancer. Mechanistically, targeting serotonin transporter suppressed mTOR serotonylation, leading to mTOR inactivation and increased tryptophan uptake. In turn, this process promoted serotonin biosynthesis and oncogenic metabolite kynurenine production through enhanced tryptophan catabolism. Tryptophan deprivation, or blocking its uptake by using trametinib, a MEK inhibitor, can sensitize colon cancer to selective serotonin reuptake inhibitors.
Conclusions: The present study elucidated a novel feedback mechanism involved in the regulation of serotonin homeostasis and suggested innovative strategies for selective serotonin reuptake inhibitors-based treatment of colon cancer.
Keywords: SERT; Serotonin; Trametinib; Tryptophan; mTORC1.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
MYC promotes tryptophan uptake and metabolism by the kynurenine pathway in colon cancer.Genes Dev. 2019 Sep 1;33(17-18):1236-1251. doi: 10.1101/gad.327056.119. Epub 2019 Aug 15. Genes Dev. 2019. PMID: 31416966 Free PMC article.
-
Down-regulating IL-6/GP130 targets improved the anti-tumor effects of 5-fluorouracil in colon cancer.Apoptosis. 2018 Jun;23(5-6):356-374. doi: 10.1007/s10495-018-1460-0. Apoptosis. 2018. PMID: 29777330
-
cGMP-dependent protein kinase Ialpha associates with the antidepressant-sensitive serotonin transporter and dictates rapid modulation of serotonin uptake.Mol Brain. 2009 Aug 5;2:26. doi: 10.1186/1756-6606-2-26. Mol Brain. 2009. PMID: 19656393 Free PMC article.
-
Gut Barrier Proteins Mediate Liver Regulation by the Effects of Serotonin on the Non-Alcoholic Fatty Liver Disease.Curr Protein Pept Sci. 2020;21(10):978-984. doi: 10.2174/1389203721666200615171928. Curr Protein Pept Sci. 2020. PMID: 32538722 Review.
-
Computational approaches for the study of serotonin and its membrane transporter SERT: implications for drug design in neurological sciences.Curr Med Chem. 2008;15(30):3214-27. doi: 10.2174/092986708786848523. Curr Med Chem. 2008. PMID: 19075665 Review.
Cited by
-
Disruption of autophagy by increased 5-HT alters gut microbiota and enhances susceptibility to experimental colitis and Crohn's disease.Sci Adv. 2021 Nov 5;7(45):eabi6442. doi: 10.1126/sciadv.abi6442. Epub 2021 Nov 5. Sci Adv. 2021. PMID: 34739317 Free PMC article.
-
KLF7 Promotes Hepatocellular Carcinoma Progression Through Regulating SLC1A5-Mediated Tryptophan Metabolism.J Cell Mol Med. 2024 Dec;28(23):e70245. doi: 10.1111/jcmm.70245. J Cell Mol Med. 2024. PMID: 39648156 Free PMC article.
-
Serotonin signalling in cancer: Emerging mechanisms and therapeutic opportunities.Clin Transl Med. 2024 Jul;14(7):e1750. doi: 10.1002/ctm2.1750. Clin Transl Med. 2024. PMID: 38943041 Free PMC article. Review.
-
Molecular mechanisms and therapeutic significance of Tryptophan Metabolism and signaling in cancer.Mol Cancer. 2024 Oct 30;23(1):241. doi: 10.1186/s12943-024-02164-y. Mol Cancer. 2024. PMID: 39472902 Free PMC article. Review.
-
Repurposing of nervous system drugs for cancer treatment: recent advances, challenges, and future perspectives.Discov Oncol. 2025 Mar 26;16(1):396. doi: 10.1007/s12672-025-02067-4. Discov Oncol. 2025. PMID: 40133751 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

