An Oncolytic Virus Expressing IL15/IL15Rα Combined with Off-the-Shelf EGFR-CAR NK Cells Targets Glioblastoma
- PMID: 34006525
- PMCID: PMC8562586
- DOI: 10.1158/0008-5472.CAN-21-0035
An Oncolytic Virus Expressing IL15/IL15Rα Combined with Off-the-Shelf EGFR-CAR NK Cells Targets Glioblastoma
Abstract
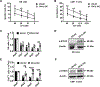
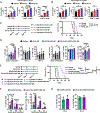
IL15 is a pleiotropic cytokine with multiple roles that improve immune responses to tumor cells. Oncolytic viruses (OV) specifically lyse tumors and activate immune responses. Systemic administration of IL15 or its complex with the IL15Rα and chimeric antigen receptor (CAR) natural killer (NK) cells are currently being tested in the clinic. Here, we generated a herpes simplex 1-based OV-expressing human IL15/IL15Rα sushi domain fusion protein (named OV-IL15C), as well as off-the-shelf EGFR-CAR NK cells, and studied their monotherapy and combination efficacy in vitro and in multiple glioblastoma (GBM) mouse models. In vitro, soluble IL15/IL15Rα complex was secreted from OV-IL15C-infected GBM cells, which promoted GBM cytotoxicity and improved survival of NK and CD8+ T cells. Frozen, readily available off-the-shelf EGFR-CAR NK cells showed enhanced killing of tumor cells compared with empty vector-transduced NK cells. In vivo, OV-IL15C significantly inhibited tumor growth and prolonged survival of GBM-bearing mice in the presence of CD8+ T cells compared with parental OV. OV-IL15C plus EGFR-CAR NK cells synergistically suppressed tumor growth and significantly improved survival compared with either monotherapy, correlating with increased intracranial infiltration and activation of NK and CD8+ T cells and elevated persistence of CAR NK cells in an immunocompetent model. Collectively, OV-IL15C and off-the-shelf EGFR-CAR NK cells represent promising therapeutic strategies for GBM treatment to improve the clinical management of this devastating disease. SIGNIFICANCE: The combination of an oncolytic virus expressing the IL15/IL15Rα complex and frozen, ready-to-use EGFR-CAR NK cells elicits strong antitumor responses in glioblastoma.
©2021 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest: M.A. Caligiuri and J. Yu are co-founders of CytoImmune, Inc.
Figures





References
-
- Todo T, Rabkin SD, Sundaresan P, Wu A, Meehan KR, Herscowitz HB, et al. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum Gene Ther 1999;10:2741–55 - PubMed
-
- Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol 1999;17:19–49 - PubMed
-
- Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity 2002;17:537–47 - PubMed
-
- Van den Bergh JMJ, Lion E, Van Tendeloo VFI, Smits ELJM. IL-15 receptor alpha as the magic wand to boost the success of IL-15 antitumor therapies: The upswing of IL-15 transpresentation. Pharmacology & Therapeutics 2017;170:73–9 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

