Prospective bladder cancer infrastructure for experimental and observational research on bladder cancer: study protocol for the 'trials within cohorts' study ProBCI
- PMID: 34006553
- PMCID: PMC8130738
- DOI: 10.1136/bmjopen-2020-047256
Prospective bladder cancer infrastructure for experimental and observational research on bladder cancer: study protocol for the 'trials within cohorts' study ProBCI
Abstract
Introduction: A better understanding of the molecular profile of bladder tumours, the identification of novel therapeutic targets, and introduction of new drugs and has renewed research interest in the field of bladder cancer. We describe the design and setup of a Dutch Prospective Bladder Cancer Infrastructure (ProBCI) as a means to stimulate and accelerate clinically meaningful experimental and observational research.
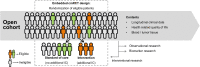
Methods and analysis: ProBCI entails an open cohort of patients with bladder cancer in which the trials within cohorts (TwiCs) design can be embedded. Physicians in participating hospitals prospectively recruit invasive (≥T1) patients with bladder cancer on primary diagnosis for inclusion into the study. Extensive clinical data are collected and updated every 4 months, along with patient-reported outcomes and biomaterials. Informed consent includes participation in TwiCs studies and renewed contact for future studies. Consent for participation in questionnaires and molecular analyses that may yield incidental findings is optional.
Ethics and dissemination: The Dutch ProBCI is a unique effort to construct a nation-wide cohort of patients with bladder cancer including clinical data, patient-reported outcomes and biomaterial, to facilitate observational and experimental research. Data and materials are available for other research groups on request through www.probci.nl. Ethics approval was obtained from METC Utrecht (reference: NL70207.041.19).
Trial registration number: NCT04503577.
Keywords: epidemiology; oncology; urological tumours.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: NM reports grants and personal fees from Merck Sharp & Dohme, Roche, Janssen, AstraZeneca, grants from Sanofi and Pfizer, and personal fees from Astellas Pharma, Bristol-Myers Squibb, and Bayer outside the submitted work. JLB reports personal fees from Merck Sharp & Dohme, Roche and Janssen outside the submitted work. AGvdH reports personal fees from Merck Sharp & Dohme, Roche, Janssen, Sanofi, Ipsen Farmacuetica, Astellas Pharma and MML outside the submitted work. MSvdH reports grants and personal fees from Roche, Bristol-Myers Squibb and AstraZeneca, and personal fees from Merck Sharp & Dohme, Janssen, Astellas Pharma and Seattle Genetics outside the submitted work. AR, KKA, RPM and LAK have nothing to disclose.
Figures
Similar articles
-
The PRO-RCC study: a long-term PROspective Renal Cell Carcinoma cohort in the Netherlands, providing an infrastructure for 'Trial within Cohorts' study designs.BMC Cancer. 2023 Jul 11;23(1):648. doi: 10.1186/s12885-023-11094-9. BMC Cancer. 2023. PMID: 37434119 Free PMC article.
-
Graham Roberts Study protocol: first 'trials within cohort study' for bladder cancer.BMJ Open. 2019 Sep 26;9(9):e029468. doi: 10.1136/bmjopen-2019-029468. BMJ Open. 2019. PMID: 31558452 Free PMC article.
-
Insight into bladder cancer care: study protocol of a large nationwide prospective cohort study (BlaZIB).BMC Cancer. 2020 May 20;20(1):455. doi: 10.1186/s12885-020-06954-7. BMC Cancer. 2020. PMID: 32434491 Free PMC article.
-
New horizons in bladder cancer research.Urol Oncol. 2020 Dec;38(12):867-885. doi: 10.1016/j.urolonc.2018.12.014. Epub 2019 Mar 7. Urol Oncol. 2020. PMID: 30852032 Review.
-
Oncology patients were found to understand and accept the Trials within Cohorts design.J Clin Epidemiol. 2021 Feb;130:135-142. doi: 10.1016/j.jclinepi.2020.10.015. Epub 2020 Oct 31. J Clin Epidemiol. 2021. PMID: 33130236 Review.
Cited by
-
Treatment Patterns and Use of Immune Checkpoint Inhibitors Among Patients with Metastatic Bladder Cancer in a Dutch Nationwide Cohort.Eur Urol Open Sci. 2023 Dec 20;59:50-54. doi: 10.1016/j.euros.2023.11.010. eCollection 2024 Jan. Eur Urol Open Sci. 2023. PMID: 38213646 Free PMC article.
-
Randomised trials conducted using cohorts: a scoping review.BMJ Open. 2024 Mar 8;14(3):e075601. doi: 10.1136/bmjopen-2023-075601. BMJ Open. 2024. PMID: 38458814 Free PMC article.
-
The PRO-RCC study: a long-term PROspective Renal Cell Carcinoma cohort in the Netherlands, providing an infrastructure for 'Trial within Cohorts' study designs.BMC Cancer. 2023 Jul 11;23(1):648. doi: 10.1186/s12885-023-11094-9. BMC Cancer. 2023. PMID: 37434119 Free PMC article.
-
The impact of positive surgical margins after cystectomy on oncological outcomes: a nationwide study.BJU Int. 2025 May;135(5):766-774. doi: 10.1111/bju.16611. Epub 2024 Dec 4. BJU Int. 2025. PMID: 39631746 Free PMC article.
-
The Trial within Cohorts (TwiCs) study design in oncology: experience and methodological reflections.BMC Med Res Methodol. 2023 May 13;23(1):117. doi: 10.1186/s12874-023-01941-5. BMC Med Res Methodol. 2023. PMID: 37179306 Free PMC article.
References
-
- Cambier S, Sylvester RJ, Collette L, et al. Eortc nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance Bacillus Calmette-Guérin. Eur Urol 2016;69:60–9. 10.1016/j.eururo.2015.06.045 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical