Region-Specific and State-Dependent Astrocyte Ca2+ Dynamics during the Sleep-Wake Cycle in Mice
- PMID: 34006590
- PMCID: PMC8221592
- DOI: 10.1523/JNEUROSCI.2912-20.2021
Region-Specific and State-Dependent Astrocyte Ca2+ Dynamics during the Sleep-Wake Cycle in Mice
Abstract
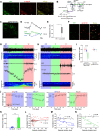
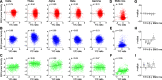
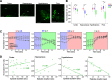
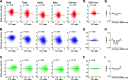
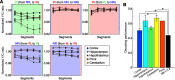
Neural activity is diverse, and varies depending on brain regions and sleep/wakefulness states. However, whether astrocyte activity differs between sleep/wakefulness states, and whether there are differences in astrocyte activity among brain regions remain poorly understood. Therefore, in this study, we recorded astrocyte intracellular calcium (Ca2+) concentrations of mice during sleep/wakefulness states in the cortex, hippocampus, hypothalamus, cerebellum, and pons using fiber photometry. For this purpose, male transgenic mice expressing the genetically encoded ratiometric Ca2+ sensor YCnano50 specifically in their astrocytes were used. We demonstrated that Ca2+ levels in astrocytes substantially decrease during rapid eye movement (REM) sleep, and increase after the onset of wakefulness. In contrast, differences in Ca2+ levels during non-REM (NREM) sleep were observed among the different brain regions, and no significant decrease was observed in the hypothalamus and pons. Further analyses focusing on the transition between sleep/wakefulness states and correlation analysis with the duration of REM sleep showed that Ca2+ dynamics differs among brain regions, suggesting the existence of several clusters, i.e., the first comprising the cortex and hippocampus, the second comprising the hypothalamus and pons, and the third comprising the cerebellum. Our study thus demonstrated that astrocyte Ca2+ levels change substantially according to sleep/wakefulness states. These changes were consistent in general unlike neural activity. However, we also clarified that Ca2+ dynamics varies depending on the brain region, implying that astrocytes may play various physiological roles in sleep.SIGNIFICANCE STATEMENT Sleep is an instinctive behavior of many organisms. In the previous five decades, the mechanism of the neural circuits controlling sleep/wakefulness states and the neural activities associated with sleep/wakefulness states in various brain regions have been elucidated. However, whether astrocytes, which are a type of glial cell, change their activity during different sleep/wakefulness states was poorly understood. Here, we demonstrated that dynamic changes in astrocyte Ca2+ concentrations occur in the cortex, hippocampus, hypothalamus, cerebellum, and pons of mice during natural sleep. Further analyses demonstrated that Ca2+ dynamics slightly differ among different brain regions, implying that the physiological roles of astrocytes in sleep/wakefulness might vary depending on the brain region.
Keywords: astrocyte; calcium; sleep; wakefulness.
Copyright © 2021 the authors.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
