Intervening in Symbiotic Cross-Kingdom Biofilm Interactions: a Binding Mechanism-Based Nonmicrobicidal Approach
- PMID: 34006656
- PMCID: PMC8262967
- DOI: 10.1128/mBio.00651-21
Intervening in Symbiotic Cross-Kingdom Biofilm Interactions: a Binding Mechanism-Based Nonmicrobicidal Approach
Abstract
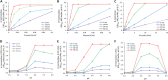
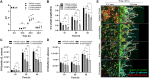
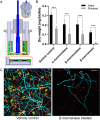
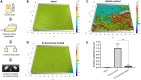
Early childhood caries is a severe oral disease that results in aggressive tooth decay. Particularly, a synergistic association between a fungus, Candida albicans, and a cariogenic bacterium, Streptococcus mutans, promotes the development of hard-to-remove and highly acidic biofilms, exacerbating the virulent damage. These interactions are largely mediated via glucosyltransferases (GtfB) binding to mannans on the cell wall of C. albicans Here, we present an enzymatic approach to target GtfB-mannan interactions in this cross-kingdom consortium using mannan-degrading exo- and endo-enzymes. These exo- and endo-enzymes are highly effective in reducing biofilm biomass without killing microorganisms, as well as alleviating the production of an acidic pH environment conducive to tooth decay. To corroborate these results, we present biophysical evidence using single-molecule atomic force microscopy, biofilm shearing, and enamel surface topography analyses. Data show a drastic decrease in binding forces of GtfB to C. albicans (∼15-fold reduction) following enzyme treatment. Furthermore, enzymatic activity disrupted biofilm mechanical stability and significantly reduced human tooth enamel demineralization without cytotoxic effects on gingival keratinocytes. Our results represent significant progress toward a novel nonbiocidal therapeutic intervention against pathogenic bacterial-fungal biofilms by targeting the interkingdom receptor-ligand binding interactions.IMPORTANCE Biofilm formation is a key virulence factor responsible for various infectious diseases. Particularly, interactions between a fungus, Candida albicans, and a bacterium, Streptococcus mutans, have been known to play important roles in the pathogenesis of dental caries. Although some antimicrobials have been applied to treat fungal-involved biofilm-associated diseases, these often lack targeting polymicrobial interactions. Furthermore, these may not be appropriate for preventive measures because these antimicrobials may disrupt ecological microbiota and/or induce the prevalence of drug resistance over time. By specifically targeting the interaction mechanism whereby mannoproteins on the C. albicans surface mediate the cross-kingdom interaction, we demonstrated that mannoprotein-degrading enzymes can effectively disrupt biofilm interactions without microbiocidal effects or causing cytotoxicity to human cells. This suggests a potential application as a targeted approach for intervening a pathogenic cross-kingdom biofilm associated with a costly and unresolved oral disease.
Keywords: Candida albicans; Streptococcus mutans; mannan-degrading enzymes; nonmicrobicidal approach; polymicrobial interaction.
Copyright © 2021 Kim et al.
Figures


Similar articles
-
Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo.PLoS Pathog. 2017 Jun 15;13(6):e1006407. doi: 10.1371/journal.ppat.1006407. eCollection 2017 Jun. PLoS Pathog. 2017. PMID: 28617874 Free PMC article.
-
Multi-omics Analyses Reveal Synergistic Carbohydrate Metabolism in Streptococcus mutans-Candida albicans Mixed-Species Biofilms.Infect Immun. 2019 Sep 19;87(10):e00339-19. doi: 10.1128/IAI.00339-19. Print 2019 Oct. Infect Immun. 2019. PMID: 31383746 Free PMC article.
-
Human Tooth as a Fungal Niche: Candida albicans Traits in Dental Plaque Isolates.mBio. 2023 Feb 28;14(1):e0276922. doi: 10.1128/mbio.02769-22. Epub 2023 Jan 5. mBio. 2023. PMID: 36602308 Free PMC article.
-
Multifaceted roles of Candida albicans and Streptococcus mutans in contributing to polybiofilm infections in early childhood caries.Front Cell Infect Microbiol. 2025 Jun 27;15:1625103. doi: 10.3389/fcimb.2025.1625103. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40654570 Free PMC article. Review.
-
Current and prospective therapeutic strategies: tackling Candida albicans and Streptococcus mutans cross-kingdom biofilm.Front Cell Infect Microbiol. 2023 May 11;13:1106231. doi: 10.3389/fcimb.2023.1106231. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37249973 Free PMC article. Review.
Cited by
-
A dental implant-on-a-chip for 3D modeling of host-material-pathogen interactions and therapeutic testing platforms.Lab Chip. 2022 Dec 6;22(24):4905-4916. doi: 10.1039/d2lc00774f. Lab Chip. 2022. PMID: 36382363 Free PMC article.
-
Nanozyme-Shelled Microcapsules for Targeting Biofilm Infections in Confined Spaces.Adv Healthc Mater. 2025 Mar;14(8):e2402306. doi: 10.1002/adhm.202402306. Epub 2024 Oct 14. Adv Healthc Mater. 2025. PMID: 39402785 Free PMC article.
-
Insights Into the Role of Streptococcus mutans and Candida albicans in Dental Biofilm Formation and Cariogenicity: A Literature Review.Cureus. 2025 Jun 16;17(6):e86159. doi: 10.7759/cureus.86159. eCollection 2025 Jun. Cureus. 2025. PMID: 40672011 Free PMC article. Review.
-
Robotic Microcapsule Assemblies with Adaptive Mobility for Targeted Treatment of Rugged Biological Microenvironments.ACS Nano. 2025 Jan 28;19(3):3265-3281. doi: 10.1021/acsnano.4c11686. Epub 2025 Jan 13. ACS Nano. 2025. PMID: 39803835 Free PMC article.
-
Nanozyme-Based Robotics Approach for Targeting Fungal Infection.Adv Mater. 2024 Mar;36(10):e2300320. doi: 10.1002/adma.202300320. Epub 2023 Jul 8. Adv Mater. 2024. PMID: 37141008 Free PMC article.
References
-
- Parisotto TM, Steiner-Oliveira C, Silva CMSE, Rodrigues LKA, Nobre-dos-Santos M. 2010. Early childhood caries and mutans streptococci: a systematic review. Oral Health Prev Dent 8:59–70. - PubMed
-
- Wang Y, Wang S, Wu C, Chen X, Duan Z, Xu Q, Jiang W, Xu L, Wang T, Su L, Wang Y, Chen Y, Zhang J, Huang Y, Tong S, Zhou C, Deng S, Qin N. 2019. Oral microbiome alterations associated with early childhood caries highlight the importance of carbohydrate metabolic activities. mSystems 4:e00450-19. doi:10.1128/mSystems.00450-19. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

