TRPV1 alleviates osteoarthritis by inhibiting M1 macrophage polarization via Ca2+/CaMKII/Nrf2 signaling pathway
- PMID: 34006826
- PMCID: PMC8131608
- DOI: 10.1038/s41419-021-03792-8
TRPV1 alleviates osteoarthritis by inhibiting M1 macrophage polarization via Ca2+/CaMKII/Nrf2 signaling pathway
Abstract
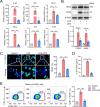
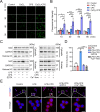
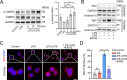
Osteoarthritis (OA) is the major course of joint deterioration, in which M1 macrophage-driven synovitis exacerbates the pathological process. However, precise therapies for M1 macrophage to decrease synovitis and attenuate OA progression have been scarcely proposed. Transient receptor potential vanilloid 1 (TRPV1) is a cation channel that has been implicated in pain perception and inflammation. In this study, we investigated the role of TRPV1 in the M1 macrophage polarization and pathogenesis of OA. We demonstrated that TRPV1 expression and M1 macrophage infiltration were simultaneously increased in both human and rat OA synovium. More than 90% of the infiltrated M1 macrophages expressed TRPV1. In the rat OA model, intra-articular injection of capsaicin (CPS), a specific TRPV1 agonist, significantly attenuated OA phenotypes, including joint swelling, synovitis, cartilage damage, and osteophyte formation. CPS treatment markedly reduced M1 macrophage infiltration in the synovium. Further mechanistic analyses showed that TRPV1-evoked Ca2+ influx promoted the phosphorylation of calcium/calmodulin-dependent protein kinase II (CaMKII) and facilitated the nuclear localization of nuclear factor-erythroid 2-related factor 2 (Nrf2), which ultimately resulted in the inhibition of M1 macrophage polarization. Taken together, our findings establish that TRPV1 attenuates the progression of OA by inhibiting M1 macrophage polarization in synovium via the Ca2+/CaMKII/Nrf2 signaling pathway. These results highlight the effect of targeting TRPV1 for the development of a promising therapeutic strategy for OA.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

