Notch3 inhibits cell proliferation and tumorigenesis and predicts better prognosis in breast cancer through transactivating PTEN
- PMID: 34006834
- PMCID: PMC8131382
- DOI: 10.1038/s41419-021-03735-3
Notch3 inhibits cell proliferation and tumorigenesis and predicts better prognosis in breast cancer through transactivating PTEN
Abstract
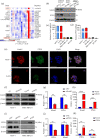
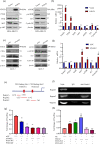
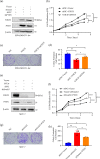
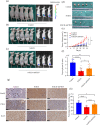
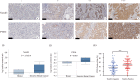
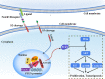
Notch receptors (Notch1-4) play critical roles in tumorigenesis and metastasis of malignant tumors, including breast cancer. Although abnormal Notch activation is related to various tumors, the importance of single receptors and their mechanism of activation in distinct breast cancer subtypes are still unclear. Previous studies by our group demonstrated that Notch3 may inhibit the emergence and progression of breast cancer. PTEN is a potent tumor suppressor, and its loss of function is sufficient to promote the occurrence and progression of tumors. Intriguingly, numerous studies have revealed that Notch1 is involved in the regulation of PTEN through its binding to CBF-1, a Notch transcription factor, and the PTEN promoter. In this study, we found that Notch3 and PTEN levels correlated with the luminal phenotype in breast cancer cell lines. Furthermore, we demonstrated that Notch3 transactivated PTEN by binding CSL-binding elements in the PTEN promoter and, at least in part, inhibiting the PTEN downstream AKT-mTOR pathway. Notably, Notch3 knockdown downregulated PTEN and promoted cell proliferation and tumorigenesis. In contrast, overexpression of the Notch3 intracellular domain upregulated PTEN and inhibited cell proliferation and tumorigenesis in vitro and in vivo. Moreover, inhibition or overexpression of PTEN partially reversed the promotion or inhibition of cell proliferation induced by Notch3 alterations. In general, Notch3 expression positively correlated with elevated expression of PTEN, ER, lower Ki-67 index, and incidence of involved node status and predicted better recurrence-free survival in breast cancer patients. Therefore, our findings demonstrate that Notch3 inhibits breast cancer proliferation and suppresses tumorigenesis by transactivating PTEN expression.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

