Small-molecule inhibition of APE1 induces apoptosis, pyroptosis, and necroptosis in non-small cell lung cancer
- PMID: 34006852
- PMCID: PMC8131371
- DOI: 10.1038/s41419-021-03804-7
Small-molecule inhibition of APE1 induces apoptosis, pyroptosis, and necroptosis in non-small cell lung cancer
Abstract
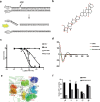
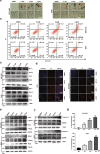
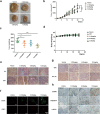
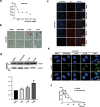
Apurinic/apyrimidinic endonuclease 1 (APE1) plays a critical role in the base excision repair (BER) pathway, which is responsible for the excision of apurinic sites (AP sites). In non-small cell lung cancer (NSCLC), APE1 is highly expressed and associated with poor patient prognosis. The suppression of APE1 could lead to the accumulation of unrepaired DNA damage in cells. Therefore, APE1 is viewed as an important marker of malignant tumors and could serve as a potent target for the development of antitumor drugs. In this study, we performed a high-throughput virtual screening of a small-molecule library using the three-dimensional structure of APE1 protein. Using the AP site cleavage assay and a cell survival assay, we identified a small molecular compound, NO.0449-0145, to act as an APE1 inhibitor. Treatment with NO.0449-0145 induced DNA damage, apoptosis, pyroptosis, and necroptosis in the NSCLC cell lines A549 and NCI-H460. This inhibitor was also able to impede cancer progression in an NCI-H460 mouse model. Moreover, NO.0449-0145 overcame both cisplatin- and erlotinib-resistance in NSCLC cell lines. These findings underscore the importance of APE1 as a therapeutic target in NSCLC and offer a paradigm for the development of small-molecule drugs that target key DNA repair proteins for the treatment of NSCLC and other cancers.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Organophosphate-pesticides induced survival mechanisms and APE1-mediated Nrf2 regulation in non-small-cell lung cancer cells.J Biochem Mol Toxicol. 2021 Feb;35(2):e22640. doi: 10.1002/jbt.22640. Epub 2020 Oct 20. J Biochem Mol Toxicol. 2021. PMID: 33078895
-
APE1 modulates cellular responses to organophosphate pesticide-induced oxidative damage in non-small cell lung carcinoma A549 cells.Mol Cell Biochem. 2018 Apr;441(1-2):201-216. doi: 10.1007/s11010-017-3186-7. Epub 2017 Sep 8. Mol Cell Biochem. 2018. PMID: 28887667
-
Small-molecule BH3 mimetic and pan-Bcl-2 inhibitor AT-101 enhances the antitumor efficacy of cisplatin through inhibition of APE1 repair and redox activity in non-small-cell lung cancer.Drug Des Devel Ther. 2015 Jun 8;9:2887-910. doi: 10.2147/DDDT.S82724. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26089640 Free PMC article.
-
Inhibitors of nuclease and redox activity of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1).Bioorg Med Chem. 2017 May 1;25(9):2531-2544. doi: 10.1016/j.bmc.2017.01.028. Epub 2017 Jan 21. Bioorg Med Chem. 2017. PMID: 28161249 Review.
-
[Multifunctional human apurinic/apyrimidinic endonuclease 1: the role of additional functions].Mol Biol (Mosk). 2007 May-Jun;41(3):450-66. Mol Biol (Mosk). 2007. PMID: 17685223 Review. Russian.
Cited by
-
Targeted Pyroptosis Is a Potential Therapeutic Strategy for Cancer.J Oncol. 2022 Nov 24;2022:2515525. doi: 10.1155/2022/2515525. eCollection 2022. J Oncol. 2022. PMID: 36467499 Free PMC article. Review.
-
The necroptosis signature and molecular mechanism of lung squamous cell carcinoma.Aging (Albany NY). 2023 Nov 15;15(22):12907-12926. doi: 10.18632/aging.205210. Epub 2023 Nov 15. Aging (Albany NY). 2023. PMID: 37976123 Free PMC article.
-
Targeting DNA Damage Repair and Immune Checkpoint Proteins for Optimizing the Treatment of Endometrial Cancer.Pharmaceutics. 2023 Aug 30;15(9):2241. doi: 10.3390/pharmaceutics15092241. Pharmaceutics. 2023. PMID: 37765210 Free PMC article. Review.
-
Inhibition of non-small cell lung cancer metastasis by knocking down APE1 through regulating myeloid-derived suppressor cells-induced immune disorders.Aging (Albany NY). 2024 Jun 14;16(12):10435-10445. doi: 10.18632/aging.205938. Epub 2024 Jun 14. Aging (Albany NY). 2024. PMID: 38885059 Free PMC article.
-
Targeting DNA Damage Response and Repair to Enhance Therapeutic Index in Cisplatin-Based Cancer Treatment.Int J Mol Sci. 2021 Jul 30;22(15):8199. doi: 10.3390/ijms22158199. Int J Mol Sci. 2021. PMID: 34360968 Free PMC article. Review.
References
-
- Wu X, et al. Cell cycle checkpoints, DNA damage/repair, and lung cancer risk. Cancer Res. 2005;65:349–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

