Diagnostic performance and characteristics of anterior nasal collection for the SARS-CoV-2 antigen test: a prospective study
- PMID: 34006975
- PMCID: PMC8131686
- DOI: 10.1038/s41598-021-90026-8
Diagnostic performance and characteristics of anterior nasal collection for the SARS-CoV-2 antigen test: a prospective study
Abstract
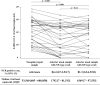
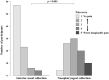
The clinical utility of antigen test using anterior nasal samples has not been well evaluated. We conducted a prospective study in a drive-through testing site located at a PCR center to evaluate the diagnostic performance of the antigen test QuickNavi-COVID19 Ag using anterior nasal samples and to compare the degrees of coughs or sneezes induction and the severity of pain between anterior nasal collection and nasopharyngeal collection. The study included a total of 862 participants, of which 91.6% were symptomatic. The median duration from symptom onset to sample collection was 2.0 days. Fifty-one participants tested positive for severe acute respiratory syndrome coronavirus 2 on reverse transcription PCR (RT-PCR) with nasopharyngeal samples, and all of them were symptomatic. In comparison to the findings of RT-PCR, the antigen test using anterior nasal samples showed 72.5% sensitivity (95% confidence interval [CI] 58.3-84.1%) and 100% specificity (95% CI 99.3-100%). Anterior nasal collection was associated with a significantly lower degree of coughs or sneezes induction and the severity of pain in comparison to nasopharyngeal collection (p < 0.001). The antigen test using anterior nasal samples showed moderate sensitivity in symptomatic patients who were at the early stages of the disease course but was less painful and induced fewer coughs or sneezes.
Conflict of interest statement
Denka Co., Ltd., provided fees for research expenses and the QuickNavi-COVID19 Ag kits without charge. H.S. received a lecture fee from Otsuka Pharmaceutical Co., Ltd., regarding this study. D.K., M.K. and S.M. are employees of Denka Co., Ltd., the developer of the QuickNavi-COVID19 Ag. All other authors declare no potential conflict of interest.
Figures

References
-
- World Health Organization. Diagnostic Testing for SARS-CoV-2: Interim Guidance, 11 September 2020. https://apps.who.int/iris/handle/10665/334254 (2020).
-
- World Health Organization. Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays: Interim Guidance, 11 September 2020. https://apps.who.int/iris/handle/10665/334253 (2020).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

