Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19
- PMID: 34007070
- PMCID: PMC8205852
- DOI: 10.1038/s41591-021-01370-1
Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19
Abstract
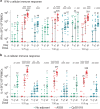
Several severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines are being deployed, but the global need greatly exceeds the supply, and different formulations might be required for specific populations. Here we report Day 42 interim safety and immunogenicity data from an observer-blinded, dose escalation, randomized controlled study of a virus-like particle vaccine candidate produced in plants that displays the SARS-CoV-2 spike glycoprotein (CoVLP: NCT04450004 ). The co-primary outcomes were the short-term tolerability/safety and immunogenicity of CoVLP formulations assessed by neutralizing antibody (NAb) and cellular responses. Secondary outcomes in this ongoing study include safety and immunogenicity assessments up to 12 months after vaccination. Adults (18-55 years, n = 180) were randomized at two sites in Quebec, Canada, to receive two intramuscular doses of CoVLP (3.75 μg, 7.5 μg, and 15 μg) 21 d apart, alone or adjuvanted with AS03 or CpG1018. All formulations were well tolerated, and adverse events after vaccination were generally mild to moderate, transient and highest in the adjuvanted groups. There was no CoVLP dose effect on serum NAbs, but titers increased significantly with both adjuvants. After the second dose, NAbs in the CoVLP + AS03 groups were more than tenfold higher than titers in Coronavirus 2019 convalescent sera. Both spike protein-specific interferon-γ and interleukin-4 cellular responses were also induced. This pre-specified interim analysis supports further evaluation of the CoVLP vaccine candidate.
Conflict of interest statement
All authors except M.P.C. either are employees of Medicago or receive salary support from Medicago (B.J.W.).
Figures



References
-
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/
-
- Wang M, et al. Evaluation of current medical approaches for COVID-19: a systematic review and meta-analysis. BMJ Support Palliat. Care. 2021;11:45–52. - PubMed
-
- Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ. 2021;372:n436. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

