Fabrication of Submicro-Nano Structures on Polyetheretherketone Surface by Femtosecond Laser for Exciting Cellular Responses of MC3T3-E1 Cells/Gingival Epithelial Cells
- PMID: 34007174
- PMCID: PMC8121686
- DOI: 10.2147/IJN.S303411
Fabrication of Submicro-Nano Structures on Polyetheretherketone Surface by Femtosecond Laser for Exciting Cellular Responses of MC3T3-E1 Cells/Gingival Epithelial Cells
Abstract
Purpose: Polyetheretherketone (PEEK) exhibits high mechanical strengths and outstanding biocompatibility but biological inertness that does not excite the cell responses and stimulate bone formation. The objective of this study was to construct submicro-nano structures on PEEK by femtosecond laser (FSL) for exciting the responses of MC3T3-E1 cells and gingival epithelial (GE) cells, which induce regeneration of bone/gingival tissues for long-term stability of dental implants.
Materials and methods: In this study, submicro-nano structures were created on PEEK surface by FSL with power of 80 mW (80FPK) and 160 mW (160FPK).
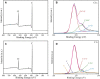
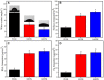
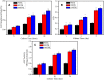
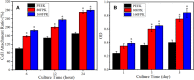
Results: Compared with PEEK, both 80FPK and 160FPK with submicro-nano structures exhibited elevated surface performances (hydrophilicity, surface energy, roughness and protein absorption). Furthermore, in comparison with 80FPK, 160FPK further enhanced the surface performances. In addition, compared with PEEK, both 80FPK and 160FPK significantly excited not only the responses (adhesion, proliferation, alkaline phosphatase [ALP] activity and osteogenic gene expression) of MC3T3-E1 cells but also responses (adhesion as well as proliferation) of GE cells of human in vitro. Moreover, in comparison with 80FPK, 160FPK further enhanced the responses of MC3T3-E1 cells/GE cells.
Conclusion: FSL created submicro-nano structures on PEEK with elevated surface performances, which played crucial roles in exciting the responses of MC3T3-E1 cells/GE cells. Consequently, 160FPK with elevated surface performances and outstanding cytocompatibility would have enormous potential as an implant for dental replacement.
Keywords: cell responses; functional group; polyetheretherketone; PEEK; submicro-nano structures; surface modification.
© 2021 Xie et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures







Similar articles
-
Nanostructured Coating of Non-Crystalline Tantalum Pentoxide on Polyetheretherketone Enhances RBMS Cells/HGE Cells Adhesion.Int J Nanomedicine. 2021 Jan 29;16:725-740. doi: 10.2147/IJN.S286643. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33542627 Free PMC article.
-
Influences of sodium tantalite submicro-particles in polyetheretherketone based composites on behaviors of rBMSCs/HGE-1 cells for dental application.Colloids Surf B Biointerfaces. 2020 Apr;188:110723. doi: 10.1016/j.colsurfb.2019.110723. Epub 2019 Dec 13. Colloids Surf B Biointerfaces. 2020. PMID: 31887651
-
Enhanced osteogenic activity of phosphorylated polyetheretherketone via surface-initiated grafting polymerization of vinylphosphonic acid.Colloids Surf B Biointerfaces. 2019 Jan 1;173:591-598. doi: 10.1016/j.colsurfb.2018.10.031. Epub 2018 Oct 13. Colloids Surf B Biointerfaces. 2019. PMID: 30352380
-
Modification of polyetheretherketone (PEEK) physical features to improve osteointegration.J Zhejiang Univ Sci B. 2022 Mar 15;23(3):189-203. doi: 10.1631/jzus.B2100622. J Zhejiang Univ Sci B. 2022. PMID: 35261215 Free PMC article. Review.
-
Application of biomolecules modification strategies on PEEK and its composites for osteogenesis and antibacterial properties.Colloids Surf B Biointerfaces. 2022 Jul;215:112492. doi: 10.1016/j.colsurfb.2022.112492. Epub 2022 Apr 12. Colloids Surf B Biointerfaces. 2022. PMID: 35430485 Review.
Cited by
-
Surface modification of polyetheretherketone for boosted osseointegration: A review.Biomater Transl. 2025 Jun 25;6(2):181-201. doi: 10.12336/bmt.24.00052. eCollection 2025. Biomater Transl. 2025. PMID: 40641993 Free PMC article. Review.
-
Research progress and future prospects of antimicrobial modified polyetheretherketone (PEEK) for the treatment of bone infections.Front Bioeng Biotechnol. 2023 Aug 3;11:1244184. doi: 10.3389/fbioe.2023.1244184. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37600311 Free PMC article. Review.
-
Small extracellular vesicles with nanomorphology memory promote osteogenesis.Bioact Mater. 2022 Jan 12;17:425-438. doi: 10.1016/j.bioactmat.2022.01.008. eCollection 2022 Nov. Bioact Mater. 2022. PMID: 35386457 Free PMC article.
-
Influence of surface nanotopography and wettability on early phases of peri-implant soft tissue healing: an in-vivo study in dogs.BMC Oral Health. 2023 Sep 8;23(1):651. doi: 10.1186/s12903-023-03347-7. BMC Oral Health. 2023. PMID: 37684664 Free PMC article.
-
The Applicability of Nanostructured Materials in Regenerating Soft and Bone Tissue in the Oral Cavity-A Review.Biomimetics (Basel). 2024 Jun 8;9(6):348. doi: 10.3390/biomimetics9060348. Biomimetics (Basel). 2024. PMID: 38921228 Free PMC article. Review.
References
-
- Tian Y, Ding SY, Peng H, et al. Osteoblast growth behavior on porous-structure titanium surface. Appl Surf Sci. 2012;261:25–30. doi:10.1016/j.apsusc.2012.07.035 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

