Review of COVID-19 Vaccines Approved in the United States of America for Emergency Use
- PMID: 34007358
- PMCID: PMC8110223
- DOI: 10.14740/jocmr4490
Review of COVID-19 Vaccines Approved in the United States of America for Emergency Use
Erratum in
-
Correction to: Gender-Based Differences in Abdominal Aortic Aneurysm Rupture: A Retrospective Study; Review of COVID-19 Vaccines Approved in the United States of America for Emergency Use; Review of COVID-19 Variants and COVID-19 Vaccine Efficacy: What the Clinician Should Know?J Clin Med Res. 2021 Jul;13(7):412. doi: 10.14740/jocmr4376c1. Epub 2021 Jul 28. J Clin Med Res. 2021. PMID: 34394785 Free PMC article.
Abstract
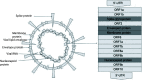
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus causing a global pandemic. Coronaviruses are a large family of single-stranded ribonucleic acid (RNA) viruses. The virus has four essential structural proteins which include the spike (S) glycoprotein, matrix (M) protein, nucleocapsid (N) protein and small envelope (E) protein. Different technologies are being used for vaccine development to battle the pandemic. There are messenger ribonucleic acid (mRNA)-based vaccines, deoxyribonucleic acid (DNA) vaccines, inactivated viral vaccines, live attenuated vaccines, protein subunit-based vaccines, viral vector-based vaccines and virus-like particle-based vaccines. Vaccine development has five stages. In the clinical developmental stage, vaccine development can be sped up by combining phase 1 and 2. The vaccines can also be approved more swiftly on an emergent basis and released sooner for usage. The United States Food and Drug Administration (USFDA) has approved Pfizer-BioNTech, Moderna and Janssen coronavirus disease 2019 (COVID-19) vaccines for emergency use. There are other vaccines that have been approved around the world. The mRNA vaccines have been created using a novel technology and they contain a synthetically created RNA sequence of virus fragments encoding the S-protein which is injected. These vaccines have a relatively low cost of production and faster manufacturing time but can have comparatively lower immunogenicity and more than one dose of vaccine may be required. In the case of viral vector-based vaccines, genes encoding the SARS-CoV-2 S protein are isolated and following gene sequencings are introduced into the adenovirus vector. These vaccines have a relatively fast manufacturing time but the efficacy of the vaccine is variable based on the host's immune response to the viral vector. At the time of this paper, there were 81 vaccines in clinical development stage and 182 vaccines in preclinical development stage. Vaccines are an essential tool in our battle against COVID-19. Some of the COVID-19 vaccines have completed their phase III trials while many other potential vaccines are still in developmental stages. It used to take close to a decade for a vaccine to be developed and undergo rigorous testing until its production and availability to the public, but over the past year, we have seen multiple vaccines in different phases of testing against SARS-CoV-2 virus.
Keywords: COVID-19; Janssen; Moderna; Pfizer-BioNTech; SARS-CoV-2; Vaccines.
Copyright 2021, Vanaparthy et al.
Conflict of interest statement
The authors declare that they do not have a conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
