The Controversial Role of Glucose-6-Phosphate Dehydrogenase Deficiency on Cardiovascular Disease: A Narrative Review
- PMID: 34007401
- PMCID: PMC8110402
- DOI: 10.1155/2021/5529256
The Controversial Role of Glucose-6-Phosphate Dehydrogenase Deficiency on Cardiovascular Disease: A Narrative Review
Abstract
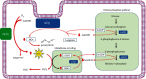
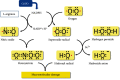
Cardiovascular disorders (CVD) are highly prevalent and the leading cause of death worldwide. Atherosclerosis is responsible for most cases of CVD. The plaque formation and subsequent thrombosis in atherosclerosis constitute an ongoing process that is influenced by numerous risk factors such as hypertension, diabetes, dyslipidemia, obesity, smoking, inflammation, and sedentary lifestyle. Among the various risk and protective factors, the role of glucose-6-phosphate dehydrogenase (G6PD) deficiency, the most common inborn enzyme disorder across populations, is still debated. For decades, it has been considered a protective factor against the development of CVD. However, in the recent years, growing scientific evidence has suggested that this inherited condition may act as a CVD risk factor. The role of G6PD deficiency in the atherogenic process has been investigated using in vitro or ex vivo cellular models, animal models, and epidemiological studies in human cohorts of variable size and across different ethnic groups, with conflicting results. In this review, the impact of G6PD deficiency on CVD was critically reconsidered, taking into account the most recent acquisitions on molecular and biochemical mechanisms, namely, antioxidative mechanisms, glutathione recycling, and nitric oxide production, as well as their mutual interactions, which may be impaired by the enzyme defect in the context of the pentose phosphate pathway. Overall, current evidence supports the notion that G6PD downregulation may favor the onset and evolution of atheroma in subjects at risk of CVD. Given the relatively high frequency of this enzyme deficiency in several regions of the world, this finding might be of practical importance to tailor surveillance guidelines and facilitate risk stratification.
Copyright © 2021 Maria Pina Dore et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

