Positive and Negative Regulation of Ferroptosis and Its Role in Maintaining Metabolic and Redox Homeostasis
- PMID: 34007410
- PMCID: PMC8102094
- DOI: 10.1155/2021/9074206
Positive and Negative Regulation of Ferroptosis and Its Role in Maintaining Metabolic and Redox Homeostasis
Abstract
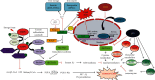
Ferroptosis is a recently recognized regulated form of cell death characterized by accumulation of lipid-based reactive oxygen species (ROS), particularly lipid hydroperoxides and loss of activity of the lipid repair enzyme glutathione peroxidase 4 (GPX4). This iron-dependent form of cell death is morphologically, biochemically, and also genetically discrete from other regulated cell death processes, which include autophagy, apoptosis, necrosis, and necroptosis. Ferroptosis is defined by three hallmarks, defined as the loss of lipid peroxide repair capacity by GPX4, the bioavailability of redox-active iron, and oxidation of polyunsaturated fatty acid- (PUFA-) containing phospholipids. Experimentally, it can be induced by many compounds (e.g., erastin, Ras-selective lethal small-molecule 3, and buthionine sulfoximine) and also can be pharmacologically inhibited by iron chelators (e.g., deferoxamine and deferoxamine mesylate) and lipid peroxidation inhibitors (e.g., ferrostatin and liproxstatin). The sensitivity of a cell towards ferroptotic cell death is tightly associated with the metabolism of amino acid, iron, and polyunsaturated fatty acid metabolism, and also with the biosynthesis of glutathione, phospholipids, NADPH, and coenzyme Q10. Ferroptosis sensitivity is also governed by many regulatory proteins, which also link ferroptosis to the function of key tumour suppressor pathways. In this review, we highlight the discovery of ferroptosis, the mechanism of ferroptosis regulation, and its association with other cellular metabolic processes.
Copyright © 2021 Ankita Sharma and Swaran Jeet Singh Flora.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

