Advancing medical technology innovation and clinical translation via a model of industry-enabled technical and educational support: Indiana Clinical and Translational Sciences Institute's Medical Technology Advance Program
- PMID: 34007464
- PMCID: PMC8111604
- DOI: 10.1017/cts.2021.1
Advancing medical technology innovation and clinical translation via a model of industry-enabled technical and educational support: Indiana Clinical and Translational Sciences Institute's Medical Technology Advance Program
Erratum in
-
Erratum: Advancing medical technology innovation and clinical translation via a model of industry-enabled technical and educational support: Indiana Clinical and Translational Sciences Institute's Medical Technology Advance Program - ERRATUM.J Clin Transl Sci. 2021 Jul 22;5(1):e131. doi: 10.1017/cts.2021.799. eCollection 2021. J Clin Transl Sci. 2021. PMID: 34367676 Free PMC article.
Abstract
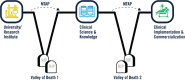
The success rate for translation of newly engineered medical technologies into clinical practice is low. Traversing the "translational valleys of death" requires a high level of knowledge of the complex landscape of technical, ethical, regulatory, and commercialization challenges along a multi-agency path of approvals. The Indiana Clinical and Translational Sciences Institute developed a program targeted at increasing that success rate through comprehensive training, education, and resourcing. The Medical Technology Advance Program (MTAP) provides technical, educational, and consultative assistance to investigators that leverages partnerships with experts in the health products industry to speed progress toward clinical implementation. The training, resourcing, and guidance are integrated through the entire journey of medical technology translation. Investigators are supported through a set of courses that cover bioethics, ethical engineering, preclinical and clinical study design, regulatory submissions, entrepreneurship, and commercialization. In addition to the integrated technical and educational resources, program experts provide direct consultation for planning each phase along the life cycle of translation. Since 2008, nearly 200 investigators have gained assistance from MTAP resulting in over 100 publications and patents. This support via medicine-engineering-industry partnership provides a unique and novel opportunity to expedite new medical technologies into clinical and product implementation.
Keywords: Translational research; academic–industry collaboration; engineering; medical technology; regulatory affairs.
© The Association for Clinical and Translational Science 2021.
Figures

References
-
- Shi Y. Clinical translation of nanomedicine and biomaterials for cancer immunotherapy: progress and perspectives. Advanced Therapeutics 2020; 3: 1900215. doi: 10.1002/adtp.201900215 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
