Development and Validation of a Robust Immune-Related Prognostic Signature for Gastric Cancer
- PMID: 34007851
- PMCID: PMC8110424
- DOI: 10.1155/2021/5554342
Development and Validation of a Robust Immune-Related Prognostic Signature for Gastric Cancer
Abstract
Background: An increasing number of reports have found that immune-related genes (IRGs) have a significant impact on the prognosis of a variety of cancers, but the prognostic value of IRGs in gastric cancer (GC) has not been fully elucidated.
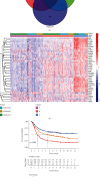
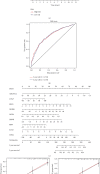
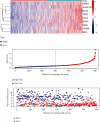
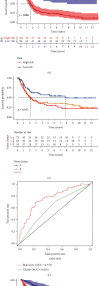
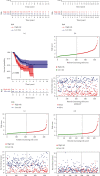
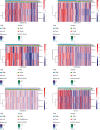
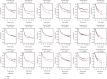
Methods: Univariate Cox regression analysis was adopted for the identification of prognostic IRGs in three independent cohorts (GSE62254, n = 300; GSE15459, n = 191; and GSE26901, n = 109). After obtaining the intersecting prognostic genes, the three independent cohorts were merged into a training cohort (n = 600) to establish a prognostic model. The risk score was determined using multivariate Cox and LASSO regression analyses. Patients were classified into low-risk and high-risk groups according to the median risk score. The risk score performance was validated externally in the three independent cohorts (GSE26253, n = 432; GSE84437, n = 431; and TCGA, n = 336). Immune cell infiltration (ICI) was quantified by the CIBERSORT method.
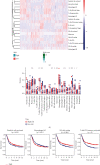
Results: A risk score comprising nine genes showed high accuracy for the prediction of the overall survival (OS) of patients with GC in the training cohort (AUC > 0.7). The risk of death was found to have a positive correlation with the risk score. The univariate and multivariate Cox regression analyses revealed that the risk score was an independent indicator of the prognosis of patients with GC (p < 0.001). External validation confirmed the universal applicability of the risk score. The low-risk group presented a lower infiltration level of M2 macrophages than the high-risk group (p < 0.001), and the prognosis of patients with GC with a higher infiltration level of M2 macrophages was poor (p = 0.011). According to clinical correlation analysis, compared with patients with the diffuse and mixed type of GC, those with the Lauren classification intestinal GC type had a significantly lower risk score (p = 0.00085). The patients' risk score increased with the progression of the clinicopathological stage.
Conclusion: In this study, we constructed and validated a robust prognostic signature for GC, which may help improve the prognostic assessment system and treatment strategy for GC.
Copyright © 2021 Junyu Huo et al.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Identification of a novel 10 immune-related genes signature as a prognostic biomarker panel for gastric cancer.Cancer Med. 2021 Sep;10(18):6546-6560. doi: 10.1002/cam4.4180. Epub 2021 Aug 12. Cancer Med. 2021. PMID: 34382341 Free PMC article.
-
Metabolism reprogramming signature associated with stromal cells abundance in tumor microenvironment improve prognostic risk classification for gastric cancer.BMC Gastroenterol. 2022 Jul 30;22(1):364. doi: 10.1186/s12876-022-02451-2. BMC Gastroenterol. 2022. PMID: 35907819 Free PMC article.
-
Integrated analysis of 1804 samples of six centers to construct and validate a robust immune-related prognostic signature associated with stromal cell abundance in tumor microenvironment for gastric cancer.World J Surg Oncol. 2022 Jan 5;20(1):4. doi: 10.1186/s12957-021-02485-y. World J Surg Oncol. 2022. PMID: 34983559 Free PMC article.
-
A novel prognostic risk score model based on immune-related genes in patients with stage IV colorectal cancer.Biosci Rep. 2020 Oct 30;40(10):BSR20201725. doi: 10.1042/BSR20201725. Biosci Rep. 2020. PMID: 33034614 Free PMC article.
-
Signature constructed by glycolysis-immune-related genes can predict the prognosis of osteosarcoma patients.Invest New Drugs. 2022 Aug;40(4):818-830. doi: 10.1007/s10637-022-01228-4. Epub 2022 Apr 18. Invest New Drugs. 2022. PMID: 35435626 Review.
Cited by
-
Comprehensive analysis of metabolic pathway activity subtypes derived prognostic signature in hepatocellular carcinoma.Cancer Med. 2023 Jan;12(1):898-912. doi: 10.1002/cam4.4858. Epub 2022 Jun 1. Cancer Med. 2023. PMID: 35651292 Free PMC article.
-
A nine-consensus-prognostic -gene-based prognostic signature, recognizing the dichotomized subgroups of gastric cancer patients with different clinical outcomes and therapeutic strategies.Front Genet. 2022 Sep 26;13:909175. doi: 10.3389/fgene.2022.909175. eCollection 2022. Front Genet. 2022. PMID: 36226177 Free PMC article.
-
Development and verification of a manganese metabolism- and immune-related genes signature for prediction of prognosis and immune landscape in gastric cancer.Front Immunol. 2024 May 13;15:1377472. doi: 10.3389/fimmu.2024.1377472. eCollection 2024. Front Immunol. 2024. PMID: 38807601 Free PMC article.
-
DNA damage repair molecular subtype derived immune signature applicable for the prognosis and immunotherapy response prediction in colon cancer.Transl Cancer Res. 2023 Oct 31;12(10):2781-2805. doi: 10.21037/tcr-23-747. Epub 2023 Oct 17. Transl Cancer Res. 2023. PMID: 37969400 Free PMC article.
-
NLRP3 inflammasome expression affects immune cell infiltration and clinical prognosis in Helicobacter pylori infection‑associated gastric cancer.Mol Med Rep. 2025 Jul;32(1):185. doi: 10.3892/mmr.2025.13550. Epub 2025 May 2. Mol Med Rep. 2025. PMID: 40314099 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

