The changing face of Australian data reforms: impact on pharmacoepidemiology research
- PMID: 34007904
- PMCID: PMC8107783
- DOI: 10.23889/ijpds.v6i1.1418
The changing face of Australian data reforms: impact on pharmacoepidemiology research
Abstract
Objective: A wealth of data is generated through Australia's universal health care arrangements. However, use of these data has been hampered by different federal and state legislation, privacy concerns and challenges in linking data across jurisdictions. A series of data reforms have been touted to increase population health research capacity in Australia, including pharmacoepidemiology research. Here we catalogued research leveraging Australia's Pharmaceutical Benefits Scheme (PBS) data (2014-2018) and discussed these outputs in the context of previously implemented and new data reforms.
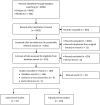
Methods: We conducted a systematic review of population-based studies using PBS dispensing claims. Independent reviewers screened abstracts of 4,996 articles and 310 full-text manuscripts. We characterised publications according to study population, analytical approach, data sources used, aims and medicines focus.
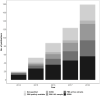
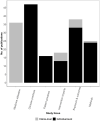
Results: We identified 180 studies; 133 used individual-level data, 70 linked PBS dispensing claims with other health data (66 across jurisdictions). Studies using individual-level data focussed on Australians receiving government benefits (87 studies) rather than all PBS-eligible persons. 63 studies examined clinician or patient practices and 33 examined exposure-outcome relationships (27 evaluated medicines safety, 6 evaluated effectiveness). Medicines acting on the nervous and cardiovascular system account for the greatest volume of PBS medicines dispensed and were the most commonly studied (67 and 40 studies, respectively). Antineoplastic and immunomodulating agents account for approximately one third of PBS expenditure but represented only 10% of studies in this review.
Conclusions: The studies in this review represent more than a third of all population-based pharmacoepidemiology research published in the last three decades in Australia. Recent data reforms have contributed to this escalating output. However, studies are concentrated among specific subpopulations and medicines classes, and there remains a limited understanding of population benefits and harms derived from medicines use. The current draft Data Availability and Transparency legislation should further bolster efforts in population health research.
Keywords: drug prescriptions; medical record linkage; observational study; pharmacoepidemiology.
Conflict of interest statement
Conflicts of interest: The authors report no actual, potential, or perceived conflict of interest with regard to the submission of this manuscript. The Centre for Big Data Research in Health, UNSW Sydney has received funding from AbbVie to conduct research, unrelated to the present study. AbbVie did not have any knowledge of, or involvement in, this study.
Figures
References
-
- Productivity Commission 2017. Data availability and use, Report No. 82,. Canberra. Available at: http://www.pc.gov.au/inquiries/completed/data-access/report
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources