Data and Safety Monitoring of COVID-19 Vaccine Clinical Trials
- PMID: 34008027
- PMCID: PMC8240876
- DOI: 10.1093/infdis/jiab263
Data and Safety Monitoring of COVID-19 Vaccine Clinical Trials
Abstract
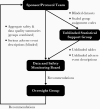
To speed the development of vaccines against SARS-CoV-2, the United States Federal Government has funded multiple phase 3 trials of candidate vaccines. A single 11-member data and safety monitoring board (DSMB) monitors all government-funded trials to ensure coordinated oversight, promote harmonized designs, and allow shared insights related to safety across trials. DSMB reviews encompass 3 domains: (1) the conduct of trials, including overall and subgroup accrual and data quality and completeness; (2) safety, including individual events of concern and comparisons by randomized group; and (3) interim analyses of efficacy when event-driven milestones are met. Challenges have included the scale and pace of the trials, the frequency of safety events related to the combined enrollment of over 100 000 participants, many of whom are older adults or have comorbid conditions that place them at independent risk of serious health events, and the politicized environment in which the trials have taken place.
Keywords: COVID19; SARS-CoV-2; clinical trials; data and safety monitoring; vaccines.
Published by Oxford University Press for the Infectious Diseases Society of America 2021.
Figures
Comment in
-
Behind the Scenes Heroes: The COVID-19 Vaccine Data and Safety Monitoring Board.J Infect Dis. 2021 Dec 15;224(12):1993-1994. doi: 10.1093/infdis/jiab267. J Infect Dis. 2021. PMID: 34007981 Free PMC article. No abstract available.
References
-
- United States Department of Health and Human Services. Trump administration announces framework and leadership for “operation warp speed.”https://www.hhs.gov/about/news/2020/05/15/trump-administration-announces.... Accessed 25 April 2021.
-
- Slaoui M, Hepburn M. Developing safe and effective covid vaccines - operation warp speed’s strategy and approach. N Engl J Med 2020; 383:1701–3. - PubMed
-
- United States Department of Health and Human Services, United States Department of Defense. From the factory to the frontlines: the operation warp speed strategy for distributing a COVID-19 vaccine, 2020. https://www.hhs.gov/sites/default/files/strategy-for-distributing-covid-.... Accessed 25 April 2021.
-
- Corey L, Mascola JR, Fauci AS, Collins FS. A strategic approach to COVID-19 vaccine R&D. Science 2020; 368:948–50. - PubMed
-
- HIV Prevention Trials Network. https://www.hptn.org/. Accessed 25 April 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

