Facile bead-to-bead cell-transfer method for serial subculture and large-scale expansion of human mesenchymal stem cells in bioreactors
- PMID: 34008349
- PMCID: PMC8380445
- DOI: 10.1002/sctm.20-0501
Facile bead-to-bead cell-transfer method for serial subculture and large-scale expansion of human mesenchymal stem cells in bioreactors
Abstract
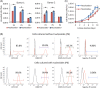
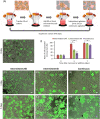
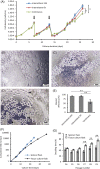
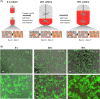
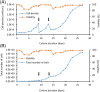
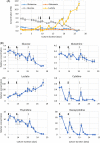
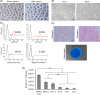
The conventional planar culture of adherent cells is inefficient for large-scale manufacturing of cell and gene therapy products. We developed a facile and efficient bead-to-bead cell-transfer method for serial subculture and large-scale expansion of human mesenchymal stem cells (hMSCs) with microcarriers in bioreactors. We first compared culture medium with and without nucleosides and found the former maintained the expression of surface markers of hMSCs during their prolonged culture and enabled faster cell proliferation. Subsequently, we developed our bead-to-bead cell transfer method to subculture hMSCs and found that intermittent agitation after adding fresh microcarriers to cell-populated microcarriers could promote spontaneous cell migration to fresh microcarriers, reduce microcarrier aggregation, and improve cell yield. This method enabled serial subculture of hMSCs in spinner flasks from passage 4 to passage 9 without using proteolytic enzymes, which showed faster cell proliferation than the serial planar cultures undergoing multiple enzyme treatment. Finally, we used the medium containing nucleosides and our bead-to-bead cell transfer method for cell culture scale-up from 4- to 50-L cultures in single-use bioreactors. We achieved a 242-fold increase in the number of cells to 1.45 × 1010 after 27-day culture and found that the cells harvested from the bioreactors maintained proliferation ability, expression of their surface markers, tri-lineage differentiation potential and immunomodulatory property. This study shows the promotive effect of nucleosides on hMSC expansion and the potential of using our bead-to-bead transfer method for larger-scale manufacturing of hMSCs for cell therapy.
Keywords: bead-to-bead transfer; bioreactor; mesenchymal stem cell; microcarrier; nucleosides; scale up.
© 2021 Showa Denko Materials Co., Ltd. STEM CELLS TRANSLATIONAL MEDICINE published by Wiley Periodicals LLC on behalf of AlphaMed Press.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures
References
-
- Kwon HM, Hur S‐M, Park K‐Y, et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascul Pharmacol. 2014;63:19‐28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

