Pioglitazone prevents obesity-related airway hyperreactivity and neuronal M2 receptor dysfunction
- PMID: 34009030
- PMCID: PMC8321847
- DOI: 10.1152/ajplung.00567.2020
Pioglitazone prevents obesity-related airway hyperreactivity and neuronal M2 receptor dysfunction
Abstract
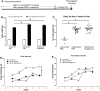
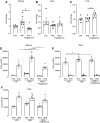
Obesity-related asthma often presents with more severe symptoms than non-obesity-related asthma and responds poorly to current treatments. Both insulin resistance and hyperinsulinemia are common in obesity. We have shown that increased insulin mediates airway hyperreactivity in diet-induced obese rats by causing neuronal M2 muscarinic receptor dysfunction, which normally inhibits acetylcholine release from parasympathetic nerves. Decreasing insulin with streptozotocin prevented airway hyperreactivity and M2 receptor dysfunction. The objective of the present study was to investigate whether pioglitazone, a hypoglycemic drug, prevents airway hyperreactivity and M2 receptor dysfunction in obese rats. Male rats fed a low- or high-fat diet were treated with pioglitazone or PBS by daily gavage. Body weight, body fat, fasting insulin, and bronchoconstriction and bradycardia in response to electrical stimulation of vagus nerves and to aerosolized methacholine were recorded. Pilocarpine, a muscarinic receptor agonist, was used to measure M2 receptor function. Rats on a high-fat diet had potentiated airway responsiveness to vagal stimulation and dysfunctional neuronal M2 receptors, whereas airway responsiveness to methacholine was unaffected. Pioglitazone reduced fasting insulin and prevented airway hyperresponsiveness and M2 receptor dysfunction but did not change inflammatory cytokine mRNA expression in alveolar macrophages. High-fat diet, with and without pioglitazone, had tissue-specific effects on insulin receptor mRNA expression. In conclusion, pioglitazone prevents vagally mediated airway hyperreactivity and protects neuronal M2 muscarinic receptor function in obese rats.
Keywords: M2 muscarinic receptors; asthma; insulin; obesity; pioglitazone.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





Similar articles
-
Metformin prevents airway hyperreactivity in rats with dietary obesity.Am J Physiol Lung Cell Mol Physiol. 2021 Dec 1;321(6):L1105-L1118. doi: 10.1152/ajplung.00202.2021. Epub 2021 Oct 20. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34668415 Free PMC article.
-
Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats.Am J Respir Cell Mol Biol. 2014 Aug;51(2):251-61. doi: 10.1165/rcmb.2013-0452OC. Am J Respir Cell Mol Biol. 2014. PMID: 24605871 Free PMC article.
-
Insulin acutely increases agonist-induced airway smooth muscle contraction in humans and rats.Am J Physiol Lung Cell Mol Physiol. 2021 Apr 1;320(4):L545-L556. doi: 10.1152/ajplung.00232.2020. Epub 2021 Jan 27. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33501891 Free PMC article.
-
Pulmonary neuronal M2 muscarinic receptor function in asthma and animal models of hyperreactivity.Thorax. 1998 Jul;53(7):613-6. doi: 10.1136/thx.53.7.613. Thorax. 1998. PMID: 9797763 Free PMC article. Review.
-
Interaction of viral infections with muscarinic receptors.Clin Exp Allergy. 1999 Jun;29 Suppl 2:59-64. doi: 10.1046/j.1365-2222.1999.00010.x. Clin Exp Allergy. 1999. PMID: 10421824 Review.
Cited by
-
Mechanisms of Obesity-related Asthma: Is Insulin Getting on Your Nerves?Am J Respir Crit Care Med. 2023 Jan 1;207(1):109-110. doi: 10.1164/rccm.202207-1419LE. Am J Respir Crit Care Med. 2023. PMID: 36029301 Free PMC article. No abstract available.
-
Eosinophil biology from the standpoint of metabolism: implications for metabolic disorders and asthma.J Leukoc Biol. 2024 Jul 25;116(2):288-296. doi: 10.1093/jleuko/qiae100. J Leukoc Biol. 2024. PMID: 38700084 Free PMC article. Review.
-
Maternal high-fat diet increases airway sensory innervation and reflex bronchoconstriction in adult offspring.Am J Physiol Lung Cell Mol Physiol. 2023 Jul 1;325(1):L66-L73. doi: 10.1152/ajplung.00115.2023. Epub 2023 Jun 6. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 37280517 Free PMC article.
-
Metformin prevents airway hyperreactivity in rats with dietary obesity.Am J Physiol Lung Cell Mol Physiol. 2021 Dec 1;321(6):L1105-L1118. doi: 10.1152/ajplung.00202.2021. Epub 2021 Oct 20. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34668415 Free PMC article.
-
Obesity-Associated Non-T2 Mechanisms in Obese Asthmatic Individuals.Biomedicines. 2023 Oct 16;11(10):2797. doi: 10.3390/biomedicines11102797. Biomedicines. 2023. PMID: 37893170 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention. Current Asthma Prevalence by Weight Status Among Adults: United States, 2001–2014. NCHS Data Brief No. 239, March 2016. https://www.cdc.gov/nchs/products/databriefs/db239.htm. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

