Insulin glargine 300 U/ml for the treatment of feline diabetes mellitus
- PMID: 34009061
- PMCID: PMC10812176
- DOI: 10.1177/1098612X211013018
Insulin glargine 300 U/ml for the treatment of feline diabetes mellitus
Abstract
Objectives: The study aimed to evaluate the efficacy and safety of insulin glargine 300 U/ml (IGla-U300) in cats with variable duration of diabetes mellitus (DM).
Methods: Thirteen client-owned cats with DM completed a prospective clinical trial. Four cats were highly suspected of hypersomatotropism and excluded from the insulin efficacy evaluation. All cats were treated with IGla-U300 SC at a starting dosage of 0.5 U/kg q12h and fed with a low carbohydrate diet. Cats were monitored for 8 weeks with a once-weekly at-home 16 h blood glucose curve (BGC) and a questionnaire evaluating the presence of DM-related clinical signs. In-clinic evaluations, including serum fructosamine measurement, were scheduled within 3 days of the first, third, sixth and eighth BGC. Glycemic variability was assessed by calculating the SD of each BGC.
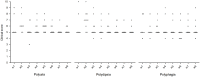
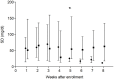
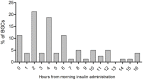
Results: Excluding four cats suspected of hypersomatotropism, at the time of the eighth BGC, improved or absent polyuria, polydipsia, polyphagia, weight loss, lethargy and improved or normal general demeanor were reported in 8/9 (88%), 8/9 (88%), 7/9 (77%), 7/9 (77%), 7/9 (77%) and 8/9 (88%) cats, respectively. Two cats achieved remission after 29 and 53 days. Another two cats went into remission after the end of the study (days 82 and 96). All cats that achieved remission were newly diagnosed diabetics. Median (range) serum fructosamine concentration significantly decreased when comparing the time of enrollment (604 [457-683] µmol/l) with the eighth week of treatment (366 [220-738] µmol/l) (P = 0.02). In all 13 cats, biochemical hypoglycemia (blood glucose <60 mg/dl; <3.3 mmol/l) was detected in 13/104 (12.5%) BGCs, while clinical signs suggesting hypoglycemic episodes were not reported. Glycemic variability was significantly lower at the fifth BGC when comparing cats that achieved remission with cats that did not achieve remission (P = 0.02).
Conclusions and relevance: IGla-U300 seems effective and safe for the treatment of feline diabetes, but more long- term and comparative clinical trials are needed.
Keywords: Glucose; blood glucose curve; fructosamine; remission; therapy.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Behrend E, Holford A, Lathan P, et al. 2018 AAHA diabetes management guidelines for dogs and cats. J Am Anim Hosp Assoc 2018; 54: 1–21. - PubMed
-
- Drugs@FDA. FDA-approved drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview... (February 25, 2015, accessed September 20, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

