Alveolar Bone Marrow Gli1+ Stem Cells Support Implant Osseointegration
- PMID: 34009063
- PMCID: PMC8721727
- DOI: 10.1177/00220345211013722
Alveolar Bone Marrow Gli1+ Stem Cells Support Implant Osseointegration
Abstract
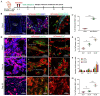
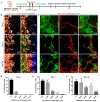
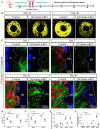
Osseointegration is the key issue for implant success. The in vivo properties of cell populations driving the osseointegration process have remained largely unknown. In the current study, using tissue clearing-based 3-dimensional imaging and transgenic mouse model-based lineage tracing methods, we identified Gli1+ cells within alveolar bone marrow and their progeny as the cell population participating in extraction socket healing and implant osseointegration. These Gli1+ cells are surrounding blood vessels and do not express lineage differentiation markers. After tooth extraction and delayed placement of a dental implant, Gli1+ cells were activated into proliferation, and their descendants contributed significantly to new bone formation. Ablation of Gli1+ cells severely compromised the healing and osseointegration processes. Blockage of canonical Wnt signaling resulted in impaired recruitment of Gli1+ cells and compromised bone healing surrounding implants. Collectively, these findings demonstrate that Gli1+ cells surrounding alveolar bone marrow vasculature are stem cells supporting dental implant osseointegration. Canonical Wnt signal plays critical roles in regulating Gli1+ stem cells.
Keywords: Wnt signaling pathway; alveolar bone; bone-implant interface; dental implantation; hedgehogs; periodontium.
Conflict of interest statement
Figures


References
-
- Branemark PI, Adell R, Breine U, Hansson BO, Lindstrom J, Ohlsson A. 1969. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 3(2):81–100. - PubMed
-
- Buser D, Sennerby L, De Bruyn H. 2017. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol; 2000. 73(1):7–21. - PubMed
-
- Calvo-Guirado JL, Ortiz-Ruiz AJ, Negri B, Lopez-Mari L, Rodriguez-Barba C, Schlottig F. 2010. Histological and histomorphometric evaluation of immediate implant placement on a dog model with a new implant surface treatment. Clin Oral Implants Res. 21(3):308–315. - PubMed
-
- Canellas JVDS, Medeiros PJD, Figueredo CMDS, Fischer RG, Ritto FG. 2019. Which is the best choice after tooth extraction, immediate implant placement or delayed placement with alveolar ridge preservation? A systematic review and meta-analysis. J Craniomaxillofac Surg. 47(11):1793–1802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

