Safety, Tolerability, and Pharmacokinetics of a Long-Acting Broadly Neutralizing Human Immunodeficiency Virus Type 1 (HIV-1) Monoclonal Antibody VRC01LS in HIV-1-Exposed Newborn Infants
- PMID: 34009371
- PMCID: PMC8643399
- DOI: 10.1093/infdis/jiab229
Safety, Tolerability, and Pharmacokinetics of a Long-Acting Broadly Neutralizing Human Immunodeficiency Virus Type 1 (HIV-1) Monoclonal Antibody VRC01LS in HIV-1-Exposed Newborn Infants
Abstract
Background: Perinatal human immunodeficiency virus type 1 (HIV-1) continues to occur due to barriers to effective antiretroviral prevention that might be mitigated by long-acting broadly neutralizing monoclonal antibodies (bNAbs).
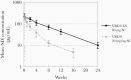
Methods: An extended half-life bNAb, VRC01LS, was administered subcutaneously at 80 mg/dose after birth to HIV-1-exposed, nonbreastfed (cohort 1, n = 10) and breastfed (cohort 2, n = 11) infants. Cohort 2 received a second dose (100 mg) at 12 weeks. All received antiretroviral prophylaxis. VRC01LS levels were compared to VRC01 levels determined in a prior cohort.
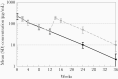
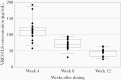
Results: Local reactions (all grade ≤2) occurred in 67% and 20% after dose 1 and dose 2, respectively. The weight-banded dose (mean 28.8 mg/kg) of VRC01LS administered subcutaneously achieved a mean (standard deviation) plasma level of 222.3 (71.6) µg/mL by 24 hours and 44.0 (11.6) µg/mL at week 12, prior to dose 2. The preestablished target of ≥50 µg/mL was attained in 95% and 32% at weeks 8 and 12, respectively. The terminal half-life was 37-41 days. VRC01LS level after 1 dose was significantly greater (P <.002) than after a VRC01 dose (20 mg/kg). No infants acquired HIV-1.
Conclusions: VRC01LS was well tolerated with pharmacokinetics that support further studies of more potent long-acting bNAbs as adjunct treatment with antiretrovirals to prevent infant HIV-1 transmission.
Keywords: HIV-1; HIV-1 prevention; VRC01; VRC01LS; bNAb; broadly neutralizing antibodies; monoclonal antibodies; neonates; passive immunization; perinatal HIV-1 transmission; pharmacokinetics.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- Gaebler C, Caskey M. Broadly neutralizing antihuman immunodeficiency virus antibodies in infants: promising new tools for prevention of mother-to-child transmission? J Infect Dis 2020; 222:525–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

