Converging Roles of the Aryl Hydrocarbon Receptor in Early Embryonic Development, Maintenance of Stemness, and Tissue Repair
- PMID: 34009372
- PMCID: PMC8285021
- DOI: 10.1093/toxsci/kfab050
Converging Roles of the Aryl Hydrocarbon Receptor in Early Embryonic Development, Maintenance of Stemness, and Tissue Repair
Abstract
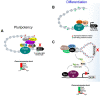
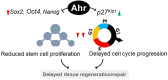
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor well-known for its adaptive role as a sensor of environmental toxicants and mediator of the metabolic detoxification of xenobiotic ligands. In addition, a growing body of experimental data has provided indisputable evidence that the AHR regulates critical functions of cell physiology and embryonic development. Recent studies have shown that the naïve AHR-that is, unliganded to xenobiotics but activated endogenously-has a crucial role in maintenance of embryonic stem cell pluripotency, tissue repair, and regulation of cancer stem cell stemness. Depending on the cellular context, AHR silences the expression of pluripotency genes Oct4 and Nanog and potentiates differentiation, whereas curtailing cellular plasticity and stemness. In these processes, AHR-mediated contextual responses and outcomes are dictated by changes of interacting partners in signaling pathways, gene networks, and cell-type-specific genomic structures. In this review, we focus on AHR-mediated changes of genomic architecture as an emerging mechanism for the AHR to regulate gene expression at the transcriptional level. Collective evidence places this receptor as a physiological hub connecting multiple biological processes whose disruption impacts on embryonic development, tissue repair, and maintenance or loss of stemness.
Keywords: Ah receptor; embryonic development; stemness; tissue repair.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society of Toxicology.All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Bersten D. C., Sullivan A. E., Peet D. J., Whitelaw M. L. (2013). BHLH-PAS proteins in cancer. Nat. Rev. Cancer 13, 827–841. - PubMed
-
- Bornelöv S., Reynolds N., Xenophontos M., Gharbi S., Johnstone E., Floyd R., Ralser M., Signolet J., Loos R., Dietmann S., et al. (2018). The nucleosome remodeling and deacetylation complex modulates chromatin structure at sites of active transcription to fine-tune gene expression. Mol. Cell 71, 56–72.e4. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

