Topologies of synthetic gene circuit for optimal fold change activation
- PMID: 34009384
- PMCID: PMC8136830
- DOI: 10.1093/nar/gkab253
Topologies of synthetic gene circuit for optimal fold change activation
Abstract
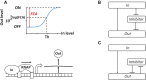
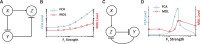
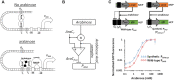
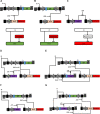
Computations widely exist in biological systems for functional regulations. Recently, incoherent feedforward loop and integral feedback controller have been implemented into Escherichia coli to achieve a robust adaptation. Here, we demonstrate that an indirect coherent feedforward loop and mutual inhibition designs can experimentally improve the fold change of promoters, by reducing the basal level while keeping the maximum activity high. We applied both designs to six different promoters in E. coli, starting with synthetic inducible promoters as a proof-of-principle. Then, we examined native promoters that are either functionally specific or systemically involved in complex pathways such as oxidative stress and SOS response. Both designs include a cascade having a repressor and a construct of either transcriptional interference or antisense transcription. In all six promoters, an improvement of up to ten times in the fold change activation was observed. Theoretically, our unitless models show that when regulation strength matches promoter basal level, an optimal fold change can be achieved. We expect that this methodology can be applied in various biological systems for biotechnology and therapeutic applications.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
Considerations for using integral feedback control to construct a perfectly adapting synthetic gene network.J Theor Biol. 2010 Oct 21;266(4):723-38. doi: 10.1016/j.jtbi.2010.07.034. Epub 2010 Aug 3. J Theor Biol. 2010. PMID: 20688080
-
A genetic mammalian proportional-integral feedback control circuit for robust and precise gene regulation.Proc Natl Acad Sci U S A. 2022 Jun 14;119(24):e2122132119. doi: 10.1073/pnas.2122132119. Epub 2022 Jun 10. Proc Natl Acad Sci U S A. 2022. PMID: 35687671 Free PMC article.
-
Expression optimization and synthetic gene networks in cell-free systems.Nucleic Acids Res. 2012 Apr;40(8):3763-74. doi: 10.1093/nar/gkr1191. Epub 2011 Dec 17. Nucleic Acids Res. 2012. PMID: 22180537 Free PMC article.
-
Potential Applications of the Escherichia coli Heat Shock Response in Synthetic Biology.Trends Biotechnol. 2018 Feb;36(2):186-198. doi: 10.1016/j.tibtech.2017.10.014. Epub 2017 Nov 7. Trends Biotechnol. 2018. PMID: 29126571 Review.
-
Towards combinatorial transcriptional engineering.Biotechnol Adv. 2017 May-Jun;35(3):390-405. doi: 10.1016/j.biotechadv.2017.03.006. Epub 2017 Mar 11. Biotechnol Adv. 2017. PMID: 28300614 Review.
Cited by
-
Combating Infectious Diseases with Synthetic Biology.ACS Synth Biol. 2022 Feb 18;11(2):528-537. doi: 10.1021/acssynbio.1c00576. Epub 2022 Jan 25. ACS Synth Biol. 2022. PMID: 35077138 Free PMC article. Review.
-
Feed-forward loop improves the transient dynamics of an antithetic biological controller.J R Soc Interface. 2025 Jan;22(222):20240467. doi: 10.1098/rsif.2024.0467. Epub 2025 Jan 22. J R Soc Interface. 2025. PMID: 39837484 Free PMC article.
-
Engineering quorum sensing-based genetic circuits enhances growth and productivity robustness of industrial E. coli at low pH.Microb Cell Fact. 2024 Sep 28;23(1):256. doi: 10.1186/s12934-024-02524-9. Microb Cell Fact. 2024. PMID: 39342182 Free PMC article.
-
Engineering coupled consortia-based biosensors for diagnostic.Nat Commun. 2025 Apr 22;16(1):3761. doi: 10.1038/s41467-025-58996-9. Nat Commun. 2025. PMID: 40263365 Free PMC article.
-
De Novo Design of the ArsR Regulated Pars Promoter Enables a Highly Sensitive Whole-Cell Biosensor for Arsenic Contamination.Anal Chem. 2022 May 24;94(20):7210-7218. doi: 10.1021/acs.analchem.2c00055. Epub 2022 May 10. Anal Chem. 2022. PMID: 35537205 Free PMC article.
References
-
- Adler M., Alon U.. Fold-change detection in biological systems. Curr. Opin. Syst. Biol. 2018; 8:81–89.
-
- Adler M., Szekely P., Mayo A., Adler M., Szekely P., Mayo A., Alon U.. Optimal regulatory circuit topologies for fold-change detection. Cell Syst. 2017; 4:171–181. - PubMed
-
- Alon U., Surette M.G., Barkai N., Leibler S.. Robustness in bacterial chemotaxis. Nature. 1999; 397:168–171. - PubMed
-
- Ferrell J.E. Previews signaling motifs and Weber’s law. Mol. Cell. 2009; 36:724–727. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

