What does elevated TARC/CCL17 expression tell us about eosinophilic disorders?
- PMID: 34009399
- PMCID: PMC8132044
- DOI: 10.1007/s00281-021-00857-w
What does elevated TARC/CCL17 expression tell us about eosinophilic disorders?
Abstract
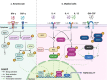
Eosinophilic disorders encompass a large spectrum of heterogeneous diseases sharing the presence of elevated numbers of eosinophils in blood and/or tissues. Among these disorders, the role of eosinophils can vary widely, ranging from a modest participation in the disease process to the predominant perpetrator of tissue damage. In many cases, eosinophilic expansion is polyclonal, driven by enhanced production of interleukin-5, mainly by type 2 helper cells (Th2 cells) with a possible contribution of type 2 innate lymphoid cells (ILC2s). Among the key steps implicated in the establishment of type 2 immune responses, leukocyte recruitment toward inflamed tissues is particularly relevant. Herein, the contribution of the chemo-attractant molecule thymus and activation-regulated chemokine (TARC/CCL17) to type 2 immunity will be reviewed. The clinical relevance of this chemokine and its target, C-C chemokine receptor 4 (CCR4), will be illustrated in the setting of various eosinophilic disorders. Special emphasis will be put on the potential diagnostic, prognostic, and therapeutic implications related to activation of the TARC/CCL17-CCR4 axis.
Keywords: C-C chemokine receptor 4 (CCR4); CCL17; Eosinophilic disorders; Eosinophils; Hypereosinophilic syndromes; Thymus and activation-regulated chemokine (TARC).
Conflict of interest statement
FR has received consultancy fees from GlaxoSmithKline and AstraZeneca, and royalties from UpToDate.
Figures
References
-
- Yamada Y, Rothenberg ME, Lee AW, Akei HS, Brandt EB, Williams DA, Cancelas JA. The FIP1L1-PDGFRA fusion gene cooperates with IL-5 to induce murine hypereosinophilic syndrome (HES)/chronic eosinophilic leukemia (CEL)–like disease. Blood. 2006;107:4071–4079. doi: 10.1182/blood-2005-08-3153. - DOI - PMC - PubMed
-
- Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, Wang J, Gray PW, Matsushima K, Yoshie O. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–88. doi: 10.1093/intimm/11.1.81. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

