Molecular, Solid-State and Surface Structures of the Conformational Polymorphic Forms of Ritonavir in Relation to their Physicochemical Properties
- PMID: 34009625
- PMCID: PMC8217055
- DOI: 10.1007/s11095-021-03048-2
Molecular, Solid-State and Surface Structures of the Conformational Polymorphic Forms of Ritonavir in Relation to their Physicochemical Properties
Abstract
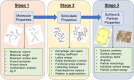
Purpose: Application of multi-scale modelling workflows to characterise polymorphism in ritonavir with regard to its stability, bioavailability and processing.
Methods: Molecular conformation, polarizability and stability are examined using quantum mechanics (QM). Intermolecular synthons, hydrogen bonding, crystal morphology and surface chemistry are modelled using empirical force fields.
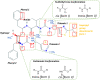
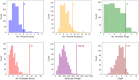
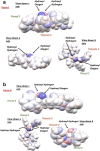
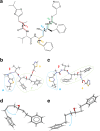
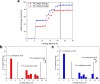
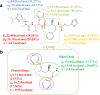
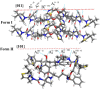
Results: The form I conformation is more stable and polarized with more efficient intermolecular packing, lower void space and higher density, however its shielded hydroxyl is only a hydrogen bond donor. In contrast, the hydroxyl in the more open but less stable and polarized form II conformation is both a donor and acceptor resulting in stronger hydrogen bonding and a more stable crystal structure but one that is less dense. Both forms have strong 1D networks of hydrogen bonds and the differences in packing energies are partially offset in form II by its conformational deformation energy difference with respect to form I. The lattice energies converge at shorter distances for form I, consistent with its preferential crystallization at high supersaturation. Both forms exhibit a needle/lath-like crystal habit with slower growing hydrophobic side and faster growing hydrophilic capping habit faces with aspect ratios increasing from polar-protic, polar-aprotic and non-polar solvents, respectively. Surface energies are higher for form II than form I and increase with solvent polarity. The higher deformation, lattice and surface energies of form II are consistent with its lower solubility and hence bioavailability.
Conclusion: Inter-relationship between molecular, solid-state and surface structures of the polymorphic forms of ritonavir are quantified in relation to their physical-chemical properties.
Keywords: Ritonavir; conformation / packing energy balance; crystal morphology; inter-molecular packing; lattice energy; molecular conformational deformation energy; particle surface energy; solvent selection; surface chemistry.
Figures


Similar articles
-
Influence of Solvent Selection on the Crystallizability and Polymorphic Selectivity Associated with the Formation of the "Disappeared" Form I Polymorph of Ritonavir.Mol Pharm. 2024 Jul 1;21(7):3525-3539. doi: 10.1021/acs.molpharmaceut.4c00234. Epub 2024 Jun 20. Mol Pharm. 2024. PMID: 38900600 Free PMC article.
-
Ritonavir: an extraordinary example of conformational polymorphism.Pharm Res. 2001 Jun;18(6):859-66. doi: 10.1023/a:1011052932607. Pharm Res. 2001. PMID: 11474792
-
Dapsone Form V: A Late Appearing Thermodynamic Polymorph of a Pharmaceutical.Mol Pharm. 2019 Jul 1;16(7):3221-3236. doi: 10.1021/acs.molpharmaceut.9b00419. Epub 2019 May 31. Mol Pharm. 2019. PMID: 31075201
-
Understanding pharmaceutical polymorphic transformations II: crystallization variables and influence on dosage forms.Ther Deliv. 2015;6(6):721-40. doi: 10.4155/tde.15.21. Ther Deliv. 2015. PMID: 26149787 Review.
-
[Crystallization and solid state properties of molecules of pharmaceutical interest].Ann Pharm Fr. 2002 May;60(3):152-60. Ann Pharm Fr. 2002. PMID: 12050594 Review. French.
Cited by
-
Influence of Solvent Selection on the Crystallizability and Polymorphic Selectivity Associated with the Formation of the "Disappeared" Form I Polymorph of Ritonavir.Mol Pharm. 2024 Jul 1;21(7):3525-3539. doi: 10.1021/acs.molpharmaceut.4c00234. Epub 2024 Jun 20. Mol Pharm. 2024. PMID: 38900600 Free PMC article.
-
Grinding Method for Phase Transformation of Glycine.ACS Omega. 2023 May 4;8(19):17116-17121. doi: 10.1021/acsomega.3c01435. eCollection 2023 May 16. ACS Omega. 2023. PMID: 37214728 Free PMC article.
-
Low-Frequency Vibrational Spectroscopy and Quantum Mechanical Simulations of the Crystalline Polymorphs of the Antiviral Drug Ribavirin.Mol Pharm. 2022 Sep 5;19(9):3385-3393. doi: 10.1021/acs.molpharmaceut.2c00509. Epub 2022 Aug 11. Mol Pharm. 2022. PMID: 35950677 Free PMC article.
-
Crystal size, shape, and conformational changes drive both the disappearance and reappearance of ritonavir polymorphs in the mill.Proc Natl Acad Sci U S A. 2024 Apr 9;121(15):e2319127121. doi: 10.1073/pnas.2319127121. Epub 2024 Apr 1. Proc Natl Acad Sci U S A. 2024. PMID: 38557191 Free PMC article.
-
Influence of Solvent Polarity on the Conformer Ratio of Bicalutamide in Saturated Solutions: Insights from NOESY NMR Analysis and Quantum-Chemical Calculations.Int J Mol Sci. 2024 Jul 28;25(15):8254. doi: 10.3390/ijms25158254. Int J Mol Sci. 2024. PMID: 39125824 Free PMC article.
References
-
- Bauer J, Spanton S, Henry R, Quick J, Dziki W, Porter W, Morris J. Ritonavir: an extraordinary example of conformational polymorphism. Pharm Res. 2001;18(6):859–866. - PubMed
-
- Cameron DW, Heath-Chiozzi M, Danner S, Cohen C, Kravcik S, Maurath C, Sun E, Henry D, Rode R, Potthoff A, Leonard J. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. Lancet. 1998;351(9102):543–549. - PubMed
-
- Markowitz M, Saag M, Powderly WG, Hurley AM, Hsu A, Valdes JM, Henry D, Sattler F, Marca AL, Leonard JM, Ho DD. A preliminary-study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med. 1995;333(23):1534–1539. - PubMed
-
- Dennington R, Keith T, Millam JJSISM, KS. GaussView, version 5. 2009.
-
- Bernstein J. Crystal polymorphism. In: Novoa JJ, Braga D, Addadi L, editors. Engineering of Crystalline Materials Properties: State of the Art in Modeling, Design and Applications. NATO Science for Peace and Security Series B-Physics and Biophysics. Dordrecht: Springer; 2008. p. 87–109.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

