Toward prevention of childhood ALL by early-life immune training
- PMID: 34010407
- PMCID: PMC8532195
- DOI: 10.1182/blood.2020009895
Toward prevention of childhood ALL by early-life immune training
Abstract
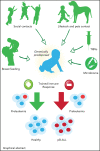
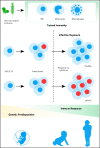
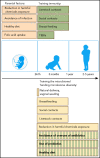
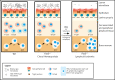
B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is the most common form of childhood cancer. Chemotherapy is associated with life-long health sequelae and fails in ∼20% of cases. Thus, prevention of leukemia would be preferable to treatment. Childhood leukemia frequently starts before birth, during fetal hematopoiesis. A first genetic hit (eg, the ETV6-RUNX1 gene fusion) leads to the expansion of preleukemic B-cell clones, which are detectable in healthy newborn cord blood (up to 5%). These preleukemic clones give rise to clinically overt leukemia in only ∼0.2% of carriers. Experimental evidence suggests that a major driver of conversion from the preleukemic to the leukemic state is exposure to immune challenges. Novel insights have shed light on immune host responses and how they shape the complex interplay between (1) inherited or acquired genetic predispositions, (2) exposure to infection, and (3) abnormal cytokine release from immunologically untrained cells. Here, we integrate the recently emerging concept of "trained immunity" into existing models of childhood BCP-ALL and suggest future avenues toward leukemia prevention.
© 2021 by The American Society of Hematology.
Figures
References
-
- Fulbright JM, Raman S, McClellan WS, August KJ.. Late effects of childhood leukemia therapy. Curr Hematol Malig Rep. 2011;6(3):195-205. - PubMed
-
- Kinlen L. Evidence for an infective cause of childhood leukaemia: comparison of a Scottish new town with nuclear reprocessing sites in Britain. Lancet. 1988;2(8624):1323-1327. - PubMed
-
- Greaves MF. Speculations on the cause of childhood acute lymphoblastic leukemia. Leukemia. 1988;2(2):120-125. - PubMed
-
- Richardson RB. Promotional etiology for common childhood acute lymphoblastic leukemia: the infective lymphoid recovery hypothesis. Leuk Res. 2011;35(11):1425-1431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

