N-Heterocyclic Carbene/Carboxylic Acid Co-Catalysis Enables Oxidative Esterification of Demanding Aldehydes/Enals, at Low Catalyst Loading
- PMID: 34010504
- PMCID: PMC8457137
- DOI: 10.1002/anie.202104712
N-Heterocyclic Carbene/Carboxylic Acid Co-Catalysis Enables Oxidative Esterification of Demanding Aldehydes/Enals, at Low Catalyst Loading
Abstract
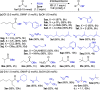
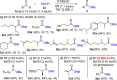
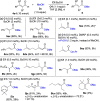
We report the discovery that simple carboxylic acids, such as benzoic acid, boost the activity of N-heterocyclic carbene (NHC) catalysts in the oxidative esterification of aldehydes. A simple and efficient protocol for the transformation of a wide range of sterically hindered α- and β-substituted aliphatic aldehydes/enals, catalyzed by a novel and readily accessible N-Mes-/N-2,4,6-trichlorophenyl 1,2,4-triazolium salt, and benzoic acid as co-catalyst, was developed. A whole series of α/β-substituted aliphatic aldehydes/enals hitherto not amenable to NHC-catalyzed esterification could be reacted at typical catalyst loadings of 0.02-1.0 mol %. For benzaldehyde, even 0.005 mol % of NHC catalyst proved sufficient: the lowest value ever achieved in NHC catalysis. Preliminary studies point to carboxylic acid-induced acceleration of acyl transfer from azolium enolate intermediates as the mechanistic basis of the observed effect.
Keywords: acylation; carbenes; cooperative catalysis; esterification; oxidation.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
1,4-Bis-Dipp/Mes-1,2,4-Triazolylidenes: Carbene Catalysts That Efficiently Overcome Steric Hindrance in the Redox Esterification of α- and β-Substituted α,β-Enals.J Am Chem Soc. 2016 Mar 2;138(8):2670-7. doi: 10.1021/jacs.5b11796. Epub 2016 Feb 22. J Am Chem Soc. 2016. PMID: 26797403
-
Acyl Donor Intermediates in N-Heterocyclic Carbene Catalysis: Acyl Azolium or Azolium Enolate?Angew Chem Int Ed Engl. 2021 Feb 23;60(9):4507-4511. doi: 10.1002/anie.202010348. Epub 2021 Jan 18. Angew Chem Int Ed Engl. 2021. PMID: 33140529 Free PMC article.
-
N-Heterocyclic Carbene (NHC)-Catalyzed Transformations Involving Azolium Enolates.Chem Rec. 2022 Aug;22(8):e202200054. doi: 10.1002/tcr.202200054. Epub 2022 May 13. Chem Rec. 2022. PMID: 35562645 Review.
-
On the mechanism of N-heterocyclic carbene-catalyzed reactions involving acyl azoliums.Acc Chem Res. 2014 Feb 18;47(2):696-707. doi: 10.1021/ar400239v. Epub 2014 Jan 10. Acc Chem Res. 2014. PMID: 24410291
-
Bifunctional N-Heterocyclic Carbenes Derived from l-Pyroglutamic Acid and Their Applications in Enantioselective Organocatalysis.Acc Chem Res. 2020 Mar 17;53(3):690-702. doi: 10.1021/acs.accounts.9b00635. Epub 2020 Mar 6. Acc Chem Res. 2020. PMID: 32142245 Review.
Cited by
-
Oxidative N-Heterocyclic Carbene Catalysis.Chemistry. 2023 Jan 18;29(4):e202202467. doi: 10.1002/chem.202202467. Epub 2022 Nov 22. Chemistry. 2023. PMID: 36205918 Free PMC article. Review.
-
Carbene and photocatalyst-catalyzed decarboxylative radical coupling of carboxylic acids and acyl imidazoles to form ketones.Nat Commun. 2022 May 23;13(1):2846. doi: 10.1038/s41467-022-30583-2. Nat Commun. 2022. PMID: 35606378 Free PMC article.
-
ThDP-dependent enzyme catalyzed oxidation of aldehydes.Chem Sci. 2025 Aug 6. doi: 10.1039/d5sc03250d. Online ahead of print. Chem Sci. 2025. PMID: 40822103 Free PMC article.
-
Cu(II) carboxylate arene C─H functionalization: Tuning for nonradical pathways.Sci Adv. 2022 Aug 26;8(34):eadd1594. doi: 10.1126/sciadv.add1594. Epub 2022 Aug 24. Sci Adv. 2022. PMID: 36001664 Free PMC article.
-
Synthesis of axially chiral diaryl ethers via NHC-catalyzed atroposelective esterification.Chem Sci. 2024 Feb 24;15(12):4564-4570. doi: 10.1039/d3sc06444a. eCollection 2024 Mar 20. Chem Sci. 2024. PMID: 38516093 Free PMC article.
References
-
- Otera J., Nishikido J., Esterification: Methods, Reactions, and Applications , 2nd ed., Wiley-VCH, Weinheim, 2010.
-
- For reviews, see:
-
- Ekoue-Kovi K., Wolf C., Chem. Eur. J. 2008, 14, 6302–6315; - PubMed
-
- Gaspa S., Porcheddu A., De Luca L., Tetrahedron Lett. 2016, 57, 3433–3440.
-
- For reviews on NHC-redox catalysis, see:
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

