GluA2 overexpression in oligodendrocyte progenitors promotes postinjury oligodendrocyte regeneration
- PMID: 34010640
- PMCID: PMC8185898
- DOI: 10.1016/j.celrep.2021.109147
GluA2 overexpression in oligodendrocyte progenitors promotes postinjury oligodendrocyte regeneration
Abstract
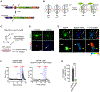
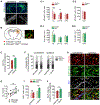
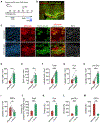
Oligodendrocyte precursor cells (OPCs) are essential for developmental myelination and oligodendrocyte regeneration after CNS injury. These progenitors express calcium-permeable AMPA receptors (AMPARs) and form direct synapses with neurons throughout the CNS, but the roles of this signaling are unclear. To enable selective alteration of the properties of AMPARs in oligodendroglia, we generate mice that allow cell-specific overexpression of EGFP-GluA2 in vivo. In healthy conditions, OPC-specific GluA2 overexpression significantly increase their proliferation in an age-dependent manner but did not alter their rate of differentiation into oligodendrocytes. In contrast, after demyelinating brain injury in neonates or adults, higher GluA2 levels promote both OPC proliferation and oligodendrocyte regeneration, but do not prevent injury-induced initial cell loss. These findings indicate that AMPAR GluA2 content regulates the proliferative and regenerative behavior of adult OPCs, serving as a putative target for better myelin repair.
Keywords: AMPA receptor; GluA2; OPC; calcium; hypoxic-ischemia; injury; oligodendrocyte; remyelination.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Bergles DE, Roberts JD, Somogyi P, and Jahr CE (2000). Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

