Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro
- PMID: 34010661
- PMCID: PMC8126375
- DOI: 10.1016/j.antiviral.2021.105089
Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro
Abstract
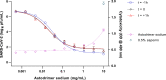
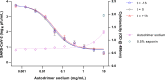
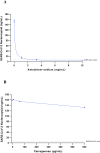
An effective response to the ongoing coronavirus disease (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will involve a range of complementary preventive modalities. The current studies were conducted to evaluate the in vitro SARS-CoV-2 antiviral and virucidal (irreversible) activity of astodrimer sodium, a dendrimer with broad spectrum antimicrobial activity, including against enveloped viruses in in vitro and in vivo models, that is marketed for antiviral and antibacterial applications. We report that astodrimer sodium inhibits replication of SARS-CoV-2 in Vero E6 and Calu-3 cells, with 50% effective concentrations (EC50) for i) reducing virus-induced cytopathic effect of 0.002-0.012 mg/mL in Vero E6 cells, and ii) infectious virus release by plaque assay of 0.019-0.032 mg/mL in Vero E6 cells and 0.030-0.037 mg/mL in Calu-3 cells. The selectivity index (SI) in these assays was as high as 2197. Astodrimer sodium was also virucidal, irreversibly reducing SARS-CoV-2 infectivity by >99.9% (>3 log10) within 1 min of exposure, and up to >99.999% (>5 log10) shown at astodrimer sodium concentrations of 10-30 mg/mL in Vero E6 and Calu-3 cell lines. Astodrimer sodium also inhibited infection in a primary human airway epithelial cell line. The data were similar for all investigations and were consistent with the potent antiviral and virucidal activity of astodrimer sodium being due to irreversible inhibition of virus-host cell interactions, as previously demonstrated for other viruses. Further studies will confirm if astodrimer sodium binds to SARS-CoV-2 spike protein and physically blocks initial attachment of the virus to the host cell. Given the in vitro effectiveness and significantly high SI, astodrimer sodium warrants further investigation for potential as a topically administered agent for SARS-CoV-2 therapeutic applications.
Keywords: Antiviral; Astodrimer; COVID-19; Dendrimer; SARS-CoV-2; SPL7013.
Copyright © 2021 Starpharma Pty Ltd. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:J.R.A.P., J.K.F. and G.P.H. are paid employees of Starpharma Pty Ltd. A.C. and C.A.L. are paid consultants to Starpharma Pty Ltd.
Figures


References
-
- Bernstein D.I., Stanberry L.R., Sacks S., Ayisi N.K., Gong Y.H., Ireland J., Mumper R.J., Holan G., Matthews B., McCarthy T., Bourne N. Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and Guinea pig models of genital herpes. Antimicrob. Agents Chemother. 2003;47(12):3784–3788. doi: 10.1128/aac.47.12.3784-3788.2003. - DOI - PMC - PubMed
-
- Chavoustie S.E., Carter B.A., Waldbaum A.S., Donders G.G.G., Peters K.H., Schwebke J.R., Paull J.R.A., Price C.F., Castellarnau A., McCloud P., Kinghorn G.R. Two phase 3, double-blinded, placebo-controlled studies of the efficacy and safety of Astodrimer 1% Gel for the treatment of bacterial vaginosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;245:13–18. doi: 10.1016/j.ejogrb.2019.11.032. - DOI - PubMed
-
- Chen M.Y., Millwood I.Y., Wand H., Poynten M., Law M., Kaldor J.M., Wesselingh S., Price C.F., Clark L.J., Paull J.R., Fairley C.K. A randomized controlled trial of the safety of candidate microbicide SPL7013 gel when applied to the penis. J. Acquir. Immune Defic. Syndr. 2009;50(4):375–380. doi: 10.1097/QAI.0b013e318198a7e6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

