Flexible, high-resolution thin-film electrodes for human and animal neural research
- PMID: 34010815
- PMCID: PMC8496685
- DOI: 10.1088/1741-2552/ac02dc
Flexible, high-resolution thin-film electrodes for human and animal neural research
Abstract
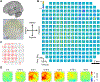
Objective.Brain functions such as perception, motor control, learning, and memory arise from the coordinated activity of neuronal assemblies distributed across multiple brain regions. While major progress has been made in understanding the function of individual neurons, circuit interactions remain poorly understood. A fundamental obstacle to deciphering circuit interactions is the limited availability of research tools to observe and manipulate the activity of large, distributed neuronal populations in humans. Here we describe the development, validation, and dissemination of flexible, high-resolution, thin-film (TF) electrodes for recording neural activity in animals and humans.Approach.We leveraged standard flexible printed-circuit manufacturing processes to build high-resolution TF electrode arrays. We used biocompatible materials to form the substrate (liquid crystal polymer; LCP), metals (Au, PtIr, and Pd), molding (medical-grade silicone), and 3D-printed housing (nylon). We designed a custom, miniaturized, digitizing headstage to reduce the number of cables required to connect to the acquisition system and reduce the distance between the electrodes and the amplifiers. A custom mechanical system enabled the electrodes and headstages to be pre-assembled prior to sterilization, minimizing the setup time required in the operating room. PtIr electrode coatings lowered impedance and enabled stimulation. High-volume, commercial manufacturing enables cost-effective production of LCP-TF electrodes in large quantities.Main Results. Our LCP-TF arrays achieve 25× higher electrode density, 20× higher channel count, and 11× reduced stiffness than conventional clinical electrodes. We validated our LCP-TF electrodes in multiple human intraoperative recording sessions and have disseminated this technology to >10 research groups. Using these arrays, we have observed high-frequency neural activity with sub-millimeter resolution.Significance.Our LCP-TF electrodes will advance human neuroscience research and improve clinical care by enabling broad access to transformative, high-resolution electrode arrays.
Keywords: Brain Machine Interface (BMI); ECoG; LCP; Neural Interface; electrode; iEEG; intraoperative.
© 2021 IOP Publishing Ltd.
Figures




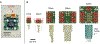


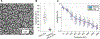



References
-
- Benabid AL et al. 2019. An exoskeleton controlled by an epidural wireless brain–machine interface in a tetraplegic patient: a proof-of-concept demonstration Lancet Neurol. 18 1112–22 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
