A randomized clinical study on the impact of Comprehensive Geriatric Assessment (CGA) based interventions on the quality of life of elderly, frail, onco-hematologic patients candidate to anticancer therapy: protocol of the ONCO-Aging study
- PMID: 34011271
- PMCID: PMC8131876
- DOI: 10.1186/s12877-021-02237-3
A randomized clinical study on the impact of Comprehensive Geriatric Assessment (CGA) based interventions on the quality of life of elderly, frail, onco-hematologic patients candidate to anticancer therapy: protocol of the ONCO-Aging study
Abstract
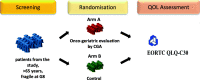
Background: Age is considered as one of the most important risk-factor for many types of solid and hematological cancers, as their incidence increases with age in parallel to the ever-growing elderly population. Moreover, cancer incidence is constantly increasing as a consequence of the increase in life expectancy that favors the process of cellular senescence. Geriatric assessment has been increasingly recognized as predictive and prognostic instrument to detect frailty in older adults with cancer. In particular, the G8 score is a simple and reproducible instrument to identify elderly patients who should undergo full geriatric evaluation. Due to their frailty, elderly patients may be often under-treated and a therapeutic choice based also on a comprehensive geriatric assessment (CGA) is recommended. With these premises, we aim to test the impact of the CGA based interventions on the quality of life (QoL) of frail elderly onco-hematological patients, identified by the G8 screening, candidate for innovative target directed drugs or treatments including the combination of radiotherapy and chemotherapy (RT + CT).
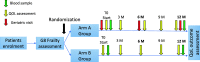
Methods: Patients aged > 65 years, candidate to target directed agents or to RT + CT treatments are screened for frailty by the G8 test; those patients classified as frail (G8 ≤ 14) are randomized to receive a CGA at baseline or to conventional care. The primary endpoint is QoL, assessed by EORTC QLQ-C30C. As collateral biological study, the potential prognostic/predictive role of T-cell senescence and myeloid derived suppressor cells (MDSC) are evaluated on plasma samples.
Discussion: This trial will contribute to define the impact of CGA on the management of frail elderly onco-hematologic patients candidate to innovative biological drugs or to integrated schedules with the association of RT + CT. Furthermore, the use of plasma samples to assess the potential prognostic value of imbalance of immune-competent cells is expected to contribute to the individualized care of elderly patients, resulting into a fine tuning of the therapeutic strategies.
Trial registration: ClinicalTrials.gov ID: NCT04478916 . registered July 21, 2020 - retrospectively registered.
Keywords: Cell senescence; Comprehensive geriatric assessment (CGA); G8 questionnaire; Quality of Life (QoL).
Conflict of interest statement
None of the authors have any conflicts of interests.
Figures


References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

