Novel mechanism for OSM-promoted extracellular matrix remodeling in breast cancer: LOXL2 upregulation and subsequent ECM alignment
- PMID: 34011405
- PMCID: PMC8132418
- DOI: 10.1186/s13058-021-01430-x
Novel mechanism for OSM-promoted extracellular matrix remodeling in breast cancer: LOXL2 upregulation and subsequent ECM alignment
Abstract
Background: Invasive ductal carcinoma (IDC) is a serious problem for patients as it metastasizes, decreasing 5-year patient survival from > 95 to ~ 27%. The breast tumor microenvironment (TME) is often saturated with proinflammatory cytokines, such as oncostatin M (OSM), which promote epithelial-to-mesenchymal transitions (EMT) in IDC and increased metastasis. The extracellular matrix (ECM) also plays an important role in promoting invasive and metastatic potential of IDC. Specifically, the reorganization and alignment of collagen fibers in stromal ECM leads to directed tumor cell motility, which promotes metastasis. Lysyl oxidase like-2 (LOXL2) catalyzes ECM remodeling by crosslinking of collagen I in the ECM. We propose a novel mechanism whereby OSM induces LOXL2 expression, mediating stromal ECM remodeling of the breast TME.
Methods: Bioinformatics was utilized to determine survival and gene correlation in patients. IDC cell lines were treated with OSM (also IL-6, LIF, and IL-1β) and analyzed for LOXL2 expression by qRT-PCR and immunolabelling techniques. Collagen I contraction assays, 3D invasion assays, and confocal microscopy were performed with and without LOXL2 inhibition to determine the impact of OSM-induced LOXL2 on the ECM.
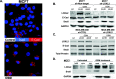
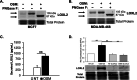
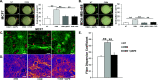
Results: Our studies demonstrate that IDC patients with high LOXL2 and OSM co-expression had worse rates of metastasis-free survival than those with high levels of either, individually, and LOXL2 expression is positively correlated to OSM/OSM receptor (OSMR) expression in IDC patients. Furthermore, human IDC cells treated with OSM resulted in a significant increase in LOXL2 mRNA, which led to upregulated protein expression of secreted, glycosylated, and enzymatically active LOXL2. The expression of LOXL2 in IDC cells did not affect OSM-promoted EMT, and LOXL2 was localized to the cytoplasm and/or secreted. OSM-induced LOXL2 promoted an increase in ECM collagen I fiber crosslinking, which led to significant fiber alignment between cells and increased IDC cell invasion.
Conclusions: Aligned collagen fibers in the ECM provide pathways for tumor cells to migrate more easily through the stroma to nearby vasculature and tissue. These results provide a new paradigm through which proinflammatory cytokine OSM promotes tumor progression. Understanding the nuances in IDC metastasis will lead to better potential therapeutics to combat against the possibility.
Keywords: Breast cancer; Collagen; Cytokines; Extracellular matrix; IL-6; Inflammation; LOXL2; Metastasis; OSM; Tumor microenvironment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review. Natl Canc Inst. 1975–2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

